![]() E331
E331
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Antioxidant (1)10 pts from Al222
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about E331 Review Consensus 10 by Al222 (23398 pt) | 2025-Sep-03 09:11 |
| Read the full Tiiip | (Send your comment) |
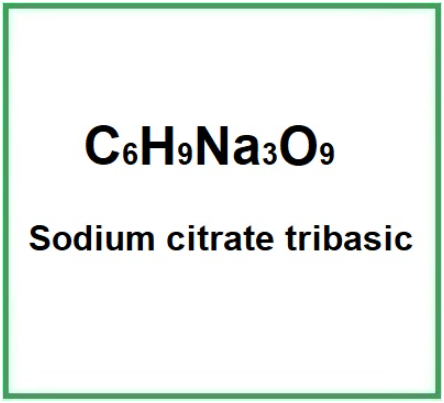
E331 or Sodium citrate tribasic or Trisodium citrate, is a chemical compound, salt of citric acid, soluble in water. It appears as small crystals or a fine, white powder.

What it is used for and where
It is used in various applications: medical, pharmaceutical, cosmetic.
Food
It is used in beverages as an acidity corrector and antioxidant.
Labeled as E331 on the food additives list as an acidity regulator to restore pH to normal, it has also been shown to improve dough in baked goods as adding sodium citrate to dough produced improved gluten aggregation and increased gluten yield over the control by raising the pH and approaching the isoelectric point of glutenin and gliadin. In addition, sodium citrate resulted in an increased particle size distribution of the glutenin macropolymer (1).
For example, in cheese, the inclusion of sodium citrate resulted in expansion of the protein matrix and increase in cheese hardness.
Medical
Citrate plays a notable role in cellular metabolism and is an important intermediate in the tricarboxylic acid cycle. In medicine it is used as an anticoagulant in stored blood, and for alkalinization of urine in the prevention of kidney stones.
In medicine, infused sodium citrate resulted in acidification and anticoagulation of blood similar to lactic acid and sodium citrate, respectively, without affecting blood gas analysis (2).
Kidney stones affect people all over the world and have a high recurrence rate even with treatment. Citrate salts prevent new stone formation and reduce further stone growth in patients with residual stones that contain predominantly oxalate (3).
The results of this study demonstrated that treatment with sodium citrate at higher concentrations or for longer periods exerts a cytotoxic effect on AGS cells through induction of the intrinsic apoptosis pathway and alteration of the levels of certain cytokines (4).
In toothpastes, the addition of sodium citrate improved dentin sensitivity (5).
Cosmetics
In cosmetics, the inclusion of sodium citrate in the formula allows to maintain the pH at acceptable levels and to regulate the overall acidity level.
Buffering agent. It is an iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
Chelating agent. It has the function of preventing unstable reactions and improving the bioavailability of chemical components within a product, and removes calcium and magnesium cations that can cause cloudiness in clear liquids.
Fragrance. It plays a decisive and important role in the formulation of cosmetic products as it provides the possibility of enhancing, masking or adding fragrance to the final product, increasing its marketability. The consumer always expects to find a pleasant or distinctive scent in a cosmetic product.
 |  |
Optimal typical characteristics of the commercial product sodium citrate
| Appearance | Small crystals or a fine, white powder. |
| Melting Point | 300°C |
| Purity | 99% |
| Density | 1.008 g/mL at 20 °C |
| Storage temperature | 2-8°C |
| Shelf life | 24 months |
Where to buy sodium citrate
- Molecular Formula : C6H5Na3O7.2H2O Na3(C6H5O7)
- Linear Formula : HOC(COONa)(CH2COONa)2 · 2H2O
- Molecular Weight : 258.068 g/mol
- CAS : 68-04-2
- EC Number : 200-675-3 213-618-2 200-675-3
- UNII RS7A450LGA
- DSSTox Substance ID: DTXSID2026363 DTXSID1027348
- MDL number MFCD00012462
- PubChem Substance ID 24899674
- InChl 1S/C6H8O7.3Na/c7-3(8)1-6(13,5(11)12)2-4(9)10;;;/h13H,1-2H2,(H,7,8)(H,9,10)(H,11,12);;;/q;3*+1/p-3
- InChI Key HRXKRNGNAMMEHJ-UHFFFAOYSA-K
- SMILES C(C(=O)[O-])C(CC(=O)[O-])(C(=O)[O-])O.[Na+].[Na+].[Na+]
- IUPAC trisodium;2-hydroxypropane-1,2,3-tricarboxylate
- ChEBI 53258
- FEMA 3026
- NACRES NA.25
Synonyms :
- Trisodium citrate
- Trisodium citrate, anhydrous
- Citrosodine
- Sodium citrate anhydrous
- Trisodium 2-hydroxy-1,2,3-propanetricarboxylate
- 1,2,3-Propanetricarboxylic acid, 2-hydroxy-, trisodium salt
References_____________________________________________________________________
(1) Amiri A, Farshi-Marandi P, Shahedi M. Impact of sodium citrate on structural properties of gluten. J Food Sci Technol. 2019 Feb;56(2):1090-1093. doi: 10.1007/s13197-019-03571-6.
Abstract. The effect of sodium citrate on gluten-starch separation and physicochemical properties of gluten was studied. The results showed that the addition of sodium citrate to the dough caused to an improvement in the aggregation of gluten and increased gluten yield in comparison to the control by augmentation pH and approaching to the isoelectric point of glutenin and gliadin. Also, sodium citrate led a shift to larger particle size distribution of glutenin macropolymer (GMP). These observations demonstrated that under the influence of sodium citrate more thiol groups were oxidized and formed disulfide bonds, which increased the storage modulus and resistance to extension. Furthermore, in this sample the GMP gel was more elastic and stiffer.
(2) Scaravilli V, Di Girolamo L, Scotti E, Busana M, Biancolilli O, Leonardi P, Carlin A, Lonati C, Panigada M, Pesenti A, Zanella A. Effects of sodium citrate, citric acid and lactic acid on human blood coagulation. Perfusion. 2018 Oct;33(7):577-583. doi: 10.1177/0267659118777441.
Abstract. Introduction: Citric acid infusion in extracorporeal blood may allow concurrent regional anticoagulation and enhancement of extracorporeal CO2 removal. Effects of citric acid on human blood thromboelastography and aggregometry have never been tested before. Methods: In this in vitro study, citric acid, sodium citrate and lactic acid were added to venous blood from seven healthy donors, obtaining concentrations of 9 mEq/L, 12 mEq/L and 15 mEq/L. We measured gas analyses, ionized calcium (iCa++) concentration, activated clotting time (ACT), thromboelastography and multiplate aggregometry. Repeated measure analysis of variance was used to compare the acidifying and anticoagulant properties of the three compounds. Results: Sodium citrate did not affect the blood gas analysis. Increasing doses of citric and lactic acid progressively reduced pH and HCO3- and increased pCO2 (p<0.001). Sodium citrate and citric acid similarly reduced iCa++, from 0.39 (0.36-0.39) and 0.35 (0.33-0.36) mmol/L, respectively, at 9 mEq/L to 0.20 (0.20-0.21) and 0.21 (0.20-0.23) mmol/L at 15 mEq/L (p<0.001). Lactic acid did not affect iCa++ (p=0.07). Sodium citrate and citric acid similarly incremented the ACT, from 234 (208-296) and 202 (178-238) sec, respectively, at 9 mEq/L, to >600 sec at 15 mEq/L (p<0.001). Lactic acid did not affect the ACT values (p=0.486). Sodium citrate and citric acid similarly incremented R-time and reduced α-angle and maximum amplitude (MA) (p<0.001), leading to flat-line thromboelastograms at 15 mEq/L. Platelet aggregometry was not altered by any of the three compounds. Conclusions: Citric acid infusions determine acidification and anticoagulation of blood similar to lactic acid and sodium citrate, respectively.
(3) Phillips R, Hanchanale VS, Myatt A, Somani B, Nabi G, Biyani CS. Citrate salts for preventing and treating calcium containing kidney stones in adults. Cochrane Database Syst Rev. 2015 Oct 6;(10):CD010057. doi: 10.1002/14651858.CD010057.pub2.
Abstract. Background: Kidney stones affect people worldwide and have a high rate of recurrence even with treatment. Recurrences are particularly prevalent in people with low urinary citrate levels. These people have a higher incidence of calcium phosphate and calcium oxalate stones. Oral citrate therapy increases the urinary citrate levels, which in turn binds with calcium and inhibits the crystallisation thus reduces stone formation. Despite the widespread use of oral citrate therapy for prevention and treatment of calcium oxalate stones, the evidence to support its clinical efficacy remains uncertain. Objectives: The objective of this review was to determine the efficacy and adverse events associated with citrate salts for the treatment and prevention of calcium containing kidney stones. Search methods: We searched the Cochrane Kidney and Transplant Specialised Register to 29 July 2015 through contact with the Trials' Search Co-ordinator using search terms relevant to this review. Selection criteria: We included randomised controlled trials (RCTs) that assessed the efficacy and adverse events associated with citrate salts for the treatment and prevention of calcium containing kidney stones in adults treated for a minimum of six months. Data collection and analysis: Two authors assessed studies for inclusion in this review. Data were extracted according to predetermined criteria. Summary estimates of effect were obtained using a random-effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes, and mean difference (MD) and 95% CI for continuous outcomes. Main results: We included seven studies that included a total of 477 participants, most of whom had oxalate stones. Of these, three studies (247 participants) compared potassium citrate with placebo or no intervention; three (166 participants) compared potassium-sodium citrate with no intervention; and one (64 participants) compared potassium-magnesium citrate with placebo. Overall, quality of the reporting of the included studies was considered moderate to poor, and there was a high risk of attrition bias in two studies.Compared with placebo or no intervention, citrate therapy significantly reduced the stone size (4 studies, 160 participants: RR 2.35, 95% CI 1.36 to 4.05). New stone formation was significantly lower with citrate therapy compared to control (7 studies, 324 participants: RR 0.26, 95% CI 0.10 to 0.68). The beneficial effect on stone size stability was also evident (4 studies, 160 participants: RR 1.97, 95% CI 1.19 to 3.26). Adverse events were reported in four studies, with the main side effects being upper gastrointestinal disturbance and one patient reported a rash. There were more gastrointestinal adverse events in the citrate group; however this was not significant (4 studies, 271 participants: RR 2.55, 95% CI 0.71 to 9.16). There were significantly more dropouts due to adverse events with citrate therapy compared to control (4 studies, 271 participants: RR 4.45, 95% CI 1.28 to 15.50). The need for retreatment was significantly less with citrate therapy compared to control (2 studies, 157 participants: RR 0.22, 95% CI 0.06 to 0.89). Authors' conclusions: Citrate salts prevent new stone formation and reduce further stone growth in patients with residual stones that predominantly contain oxalate. The quality of reported literature remains moderate to poor; hence a well-designed statistically powered multi-centre RCT is needed in order to answer relevant questions concerning the efficacy of citrate salts.
(4) Xia Y, Zhang X, Bo A, Sun J, Li M. Sodium citrate inhibits the proliferation of human gastric adenocarcinoma epithelia cells. Oncol Lett. 2018 May;15(5):6622-6628. doi: 10.3892/ol.2018.8111.
Abstract. The objective of the present study was to investigate the cytotoxic effects of sodium citrate on human gastric adenocarcinoma epithelia AGS cells. Numerous cytotoxicity-associated sodium citrate-induced effects were assessed, including cell viability and proliferation, cytokine expression and caspase activity. In vitro studies demonstrated that incubation with sodium citrate (>3.125 mM) inhibited AGS cell viability and proliferation in a dose-dependent manner. Incubation with sodium citrate for 24 h revealed that the levels of interleukin-1β (IL-1β), IL-8 and tumor necrosis factor increased with an increasing of dose of sodium citrate, whereas the IL-6 levels exhibited only a slight alteration. In addition, increases in caspase-3 and -9 activities were associated with increased duration of treatment and dosage of sodium citrate. Collectively, the results of the present study demonstrated that treatment with sodium citrate at higher concentrations or for longer durations exerts a cytotoxic effect on AGS cells via the induction of the intrinsic apoptosis pathway and the alteration in the levels of certain cytokines.
(5) Clark DC, Al-Joburi W, Chan EC. The efficacy of a new dentifrice in treating dentin sensitivity: effects of sodium citrate and sodium fluoride as active ingredients. J Periodontal Res. 1987 Mar;22(2):89-93. doi: 10.1111/j.1600-0765.1987.tb01545.x.
.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Chemical Main substances: Last update: 2023-03-22 14:45:53 | Chemical Risk: |