![]() Lysolecithin
Lysolecithin
Rating : 7.6
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Anti-inflammatory (1)10 pts from Handy23
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Lysolecithin studies" about Lysolecithin Review Consensus 7 by Handy23 (4290 pt) | 2019-Apr-22 20:26 |
| Read the full Tiiip | (Send your comment) |
Lysolecithin has been described as a powerful hemolytic. Furthermore, the toxic effect of many snake poisons is due to their content of phosphatidase A, an enzyme capable of converting plasma phosphatides into lysophosphatides, one of which is Lysolecithin (1).
An intradermal injection of 0.04 micron to 0.25 micron of Lysolecithin derived from beef serum, human serum, gave reactions with bruise and erythema in the 3 subjects tested (2).
Apart from these allergies that can be traced back to subjectivity, Lysolecithin or Lysophosphatidylcholines, has shown efficacy in protecting human skeletal muscle cells from lipotoxicity (3).
Bibliografia______________________________
(1) Safety Assessment of Lecithin and Other Phosphoglycerides as Used in Cosmetics
- September 11, 2014
(2) Middleton, E. Jr. and Phillips G. B. Release of histamine activity in human skin by lysolecithin. Laboratory and
Clinical Medicine. 1964;64(6):889-894.
(3) Lysophosphatidylcholines activate PPARδ and protect human skeletal muscle cells from lipotoxicity.
Klingler C, Zhao X, Adhikary T, Li J, Xu G, Häring HU, Schleicher E, Lehmann R, Weigert C.
Biochim Biophys Acta. 2016 Dec
(4) High-fat diet feeding differentially affects the development of inflammation in the central nervous system.
Guillemot-Legris O, Masquelier J, Everard A, Cani PD, Alhouayek M, Muccioli GG.
J Neuroinflammation. 2016 Aug
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Lysolecithin Review Consensus 10 by Handy23 (4290 pt) | 2026-Jan-08 10:06 |
| Read the full Tiiip | (Send your comment) |
Lysolecithin – naturally derived emulsifier, composition, cosmetic uses, and safety notes
Synonyms: lysolecithin, hydrolyzed lecithin, lecithins, hydrolyzed, lysophospholipid fraction from lecithin
INCI / functions: surfactant – emulsifying (co-emulsifier and emulsion stabilizer)

Definition
Lysolecithin is a functional raw material obtained via hydrolysis (often enzymatic) of lecithin, with partial conversion of phospholipids into lysophospholipids. From a compositional standpoint, the ingredient mainly contains lysophosphatidylcholine (LysoPC) and, depending on source and process, variable amounts of other lysophospholipids (e.g., lysophosphatidylethanolamine), residual non-hydrolyzed phospholipids, released fatty acids, and minor components typical of lecithins; the actual profile depends on origin (soy, sunflower, egg, or other), grade, and supplier specification. In cosmetics it is used primarily as an emulsifier/co-emulsifier and to support dispersion of oily phases in water, with potential use in delivery systems (e.g., lamellar/liposomal structures) depending on quality and formulation technology.
Food: used as an emulsifier/stabilizer (food grade; category- and specification-dependent positioning).
Cosmetics: surfactant-emulsifier, emulsion stability, sensorial and dispersion support; potential support to delivery systems (grade-dependent).
Medicine: possible technical use in formulation contexts or research (not equivalent to clinical indications).
Pharmaceutical: excipient/auxiliary for dispersing or carrier systems, subject to grade and applicable dossier.
Industrial use: surfactant/emulsifier in technical formulations and dispersion processes (sector-dependent).
The name defines the structure of the molecule
- "Lys" comes from the word "lysis", meaning splitting or breaking. In this context, it indicates that one of the acyl chains of the original lecithin has been removed.
- "Lecithin" is a generic term that refers to a variety of lipid compounds, mainly phosphatidylcholine, that are present in many plants and animals. It's a natural emulsifying agent.
Description of the raw materials used in its production.
- Lecithin. This is a natural phospholipid primarily extracted from soy or eggs. It serves as the primary precursor for the production of lysolecithin.
- Water. Used as a solvent and reaction medium.
- Enzymes. Specific enzymes, like phospholipases, are used to hydrolyze lecithin to produce lysolecithin.
Step-by-step summary of its industrial chemical synthesis process.
- Mixture Preparation. Lecithin is dissolved in water to form a solution.
- Enzymatic Hydrolysis. Enzymes are added to the lecithin solution, initiating the hydrolysis reaction.
- Separation. Once the reaction is complete, the lysolecithin is separated from the mixture.
- Purification. The lysolecithin is purified to remove any impurities.
- Drying. The purified lysolecithin is dried to remove any remaining moisture.
Calories (energy value)
| Metric | Value |
|---|---|
| Energy value (100 g) | ~900 kcal (typical order of magnitude for lipid fractions) |
| Technical note | Used at functional doses: energy impact on the finished product is negligible |
Identification data and specifications
| Parameter | Value |
|---|---|
| INCI name | Lysolecithin |
| EU database description | Lecithins, hydrolyzed |
| CAS number (cosmetic/industrial use, “hydrolyzed lecithins”) | 85711-58-6 |
| EC number | 288-318-8 |
| Note on possible alternative identifiers | Detail |
|---|---|
| “Lysolecithin” as a single molecule (laboratory use) | in literature and catalogs it may refer to specific lysophosphatidylcholines with different CAS numbers |
| Practical approach | for cosmetic use, refer to the CAS/EC of the purchased grade (lot SDS/CoA) |
Key constituents
| Class | Main components | Technical note |
|---|---|---|
| Lysophospholipids | LysoPC and analogs (grade-dependent) | primary driver of emulsifying behavior and interfacial structures |
| Residual phospholipids | phosphatidylcholine and others (variable share) | affects stability, sensorial profile, lamellar behavior |
| Fatty acids / minor components | trace fractions | can affect odor/oxidation; manage quality and storage |
| Carrier (if supplied as a solution) | water/glycerin/glycols (depending on grade) | determines dosing on active solids |
Functional role in formulation
| Function | What it does in the formula | Operational notes |
|---|---|---|
| Emulsifier / co-emulsifier | supports formation of oil-in-water emulsions and improves stability | often works synergistically with other emulsifiers and structuring lipids |
| Dispersion support | improves wetting and fineness of the dispersed phase | useful in systems with moderate lipid loads or “light” textures |
| Support to delivery systems (grade-dependent) | may promote lamellar/vesicular organization | requires dedicated development and time-stability verification |
Formulation compatibility
| System / variable | Compatibility | Control notes |
|---|---|---|
| O/W emulsions | generally good | optimize oil phase and co-emulsifiers to avoid hot/cold instability |
| Surfactant systems (rinse-off) | often compatible | verify foam, clarity, and odor |
| Electrolytes/salts | to be assessed | high salinity may influence viscosity and interfacial stability |
| Polymers/gels | sensitive | potential viscosity/clarity shifts; test at 24–48 h and under thermal cycling |
| Preservatives | to be verified | compatibility and challenge testing are essential on the finished product |
Use guidelines (indicative)
| Application | Typical range | Technical note |
|---|---|---|
| O/W creams/lotions | 0.2–2.0% | adjust based on oil phase and overall emulsifier system |
| Serums/light textures | 0.1–1.0% | useful as co-emulsifier or support for lamellar structures |
| Rinse-off cleansing | 0.1–1.0% | assess foam, stability, and surfactant compatibility |
Typical applications
Creams and lotions targeting a light texture and good spreadability.
Emulsions with improved stability and a “soft” sensorial profile.
Formulas where support to lamellar structures is desired (dedicated development).
Selected rinse-off systems as dispersion/stability support (case-by-case validation).
Quality, grades and specifications
| Parameter | Detail |
|---|---|
| Grades | cosmetic; sometimes food/technical (different specifications) |
| Recommended controls | identity and phospholipid profile, moisture, peroxide/oxidation index (if specified), microbiology (if supplied as a solution), color/odor |
| Documentation | CoA and SDS are essential to define origin, carrier, potential allergen traces, and storage requirements |
Safety, regulation and environment
| Topic | Operational guidance |
|---|---|
| Safety profile | generally suitable for cosmetic use at typical levels; manage possible individual irritation via finished-product assessment |
| Allergens / origin | if derived from soy or egg, manage potential trace considerations and supplier allergen documentation (especially for “free from” claims) |
| EU cosmetics | use under general rules and GMP; always verify finished-product dossier |
| Environment | lipid-derived material: manage effluents/residues per good practice; prevent uncontrolled release |
Formulation troubleshooting
| Issue | Possible cause | Corrective actions |
|---|---|---|
| Emulsion separation/instability | unbalanced emulsifier system or incompatible oil phase | increase co-emulsifiers, optimize structuring lipids, adjust process (shear/temperature) |
| Haze or loss of clarity | polymer interaction or high salinity | reduce electrolytes, change polymer, optimize pre-dispersion and addition order |
| Rancid odor over time | oxidation of lipid fractions | improve oxidation control (compatible antioxidants), barrier packaging, cool storage |
| Viscosity drift at 24–48 h | interfacial/lamellar reorganization | stabilize with co-structurants, verify thermal cycling and maturation |
Conclusion
Lysolecithin is a surfactant-emulsifying ingredient derived from hydrolyzed lecithin, particularly useful as a co-emulsifier in O/W emulsions and as support for dispersion and stability of aqueous systems containing lipid fractions. Performance depends critically on origin, grade, phospholipid profile, carrier (if supplied as a solution), and oxidation management, as well as on correct integration into the overall emulsifier system.
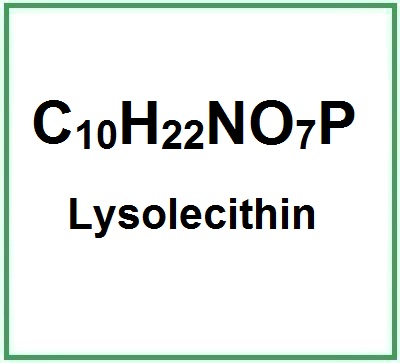
Molecular Formula: C10H22NO7P
Molecular Weight: 299.26 g/mol
CAS 85711-58-6
EC Number: 288-318-8
Synonyms:
- Lysophosphatidylcholine
- L-ALPHA-LYSOPHOSPHATIDYLCHOLINE
- 1-acetyl-sn-glycero-3-phosphocholine
- [(2R)-3-acetyloxy-2-hydroxypropyl] 2-(trimethylazaniumyl)ethyl phosphate
- 3,5,9-Trioxa-4-phosphaundecan-1-aminium, 4,7-dihydroxy-N,N,N-trimethyl-10-oxo-, inner salt, 4-oxide, (R)-
(1) Food and Drug Administration (FDA). Information supplied to FDA by industry as part of the VCRP FDA database.
2014. Washington, D.C.: FDA.
Lee HR, Kwon SY, Choi SA, Lee JH, Lee HS, Park JB. Valorization of Soy Lecithin by Enzyme Cascade Reactions Including a Phospholipase A2, a Fatty Acid Double-Bond Hydratase, and/or a Photoactivated Decarboxylase. J Agric Food Chem. 2022 Sep 7;70(35):10818-10825. doi: 10.1021/acs.jafc.2c04012.
Abstract. A huge amount of phospholipids or lecithin is produced as a byproduct in the vegetable oil industry. However, most are just used as a feed additive. This study has focused on enzymatic valorization of lecithin. This was exploited by enzymatic transformation of soy lecithin into lysolecithin liposomes, including functional free fatty acids, hydroxy fatty acids, hydrocarbons, or secondary fatty alcohols. One of the representative examples was the preparation of lysolecithin liposomes containing secondary fatty alcohols [e.g., 9-Hydroxyheptadec-11-ene (9) and 9-heptadecanol (10)] by using a phospholipase A2 from Streptomyces violaceoruber, a fatty acid double-bond hydratase from Stenotrophomonas maltophilia, and a photoactivated decarboxylase from Chlorella variabilis NC64A. The engineered liposomes turned out to range ca. 144 nm in diameter by dynamic light scattering analysis. Thereby, this study will contribute to application of functional fatty acids and their derivatives as well as valorization of lecithin for the food and cosmetic industries.
Cheng WJ, Yang HT, Chiang CC, Lai KH, Chen YL, Shih HL, Kuo JJ, Hwang TL, Lin CC. Deer Velvet Antler Extracts Exert Anti-Inflammatory and Anti-Arthritic Effects on Human Rheumatoid Arthritis Fibroblast-Like Synoviocytes and Distinct Mouse Arthritis. Am J Chin Med. 2022;50(6):1617-1643. doi: 10.1142/S0192415X22500689.
Abstract. Rheumatoid arthritis (RA) is a chronic autoimmune disease that causes joint deformity and disability. Deer velvet antler (DA), a traditional Chinese medicine, has been used to treat various types of arthritis for several thousands of years, but the underlying mechanisms are unknown. Herein, we investigated the anti-arthritic and anti-inflammatory effects of DA in vitro and in vivo. The ethyl acetate layer of DA ethanol extract (DA-EE-EA) was used to treat tumor necrosis factor (TNF)-[Formula: see text]-stimulated fibroblast-like synoviocyte MH7A cells, collagen-induced arthritis DBA/1 mice, and SKG mice with zymosan-induced arthritis. DA-EE-EA reduced nitric oxide production, prostaglandin E2 levels, and levels of pro-inflammatory cytokines including interleukin (IL)-1[Formula: see text], IL-6, and IL-8 in MH7A cells. DA-EE-EA also downregulated the phosphorylation of mitogen-activated protein kinase p38 and c-Jun N-terminal kinase and the translocation of nuclear factor kappa B p65. Intraperitoneal injection of DA-EE-EA for 3 weeks substantially reduced clinical arthritis scores in vivo models. Pathohistological images of the hind paws showed that DA-EE-EA reduced immune cell infiltration, synovial hyperplasia, and cartilage damage. The levels of pro-inflammatory cytokines, such as tumor necrosis factor alpha, IL-1[Formula: see text], IL-6, IL-8, IL-17A, and interferon-gamma, decreased in the hind paw homogenates of DA-EE-EA-treated mice. We also identified several potential components, such as hexadecanamide, oleamide, erucamide, and lysophosphatidylcholines, that might contribute to the anti-inflammatory effects of DA-EE-EA. In conclusion, DA-EE-EA has the potential to treat RA by regulating inflammatory responses. However, the individual components of DA-EE-EA and the underlying anti-inflammatory mechanisms need further investigation in future studies.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2012-11-30 19:07:56 | Chemical Risk: |