![]() DEG
DEG
Rating : 5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Cons:
To be taken in controlled quantity (1)0 pts from Al222
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about DEG by Al222 (24012 pt) | 2025-Oct-15 19:07 |
| Read the full Tiiip | (Send your comment) |
Diethylene glycol (DEG)
Diethylene glycol is an aliphatic diol (HO–CH₂–CH₂–O–CH₂–CH₂–OH), a clear, hygroscopic, faint-odor liquid with a deceptively sweet taste. It is fully miscible with water and many polar organic solvents. The empirical formula is C₄H₁₀O₃ and the molar mass is ~106.1 g/mol.
Definition and nomenclature
Common names: diethylene glycol; 2,2′-oxydiethanol; 2-(2-hydroxyethoxy)ethanol.
Abbreviation: DEG.
Related glycols: EG (monoethylene glycol), TEG (triethylene glycol).
CAS: 111-46-6.
Chemical family: low-molecular-weight polyethylene glycols (ethylene-oxide oligomers).
Physical and chemical properties (indicative)
Appearance: colorless, viscous liquid; strongly hygroscopic.
Odor/taste: almost odorless; sweet taste.
Boiling point: ~245 °C (purity and pressure dependent).
Density (20 °C): ~1.11 g/mL.
Viscosity (25 °C): on the order of tens of mPa·s.
Flash point: typically above 120 °C (closed cup, product-grade dependent).
Solubility: completely miscible with water, alcohols, ketones, and with EG/TEG.
Reactivity: terminal diol functions undergo esterification, etherification, and oxidation; acidic degradation products can form under harsh conditions.
Manufacture, commercial grades, and typical impurities
DEG is formed alongside EG and TEG during hydration of ethylene oxide and is separated by fractional distillation.
Typical impurities: EG, TEG, low-level aldehydes/acetals, water, trace metals.
Grades: technical/industrial grades and high-purity grades for specialized uses. For regulated supply chains (pharmaceuticals, cosmetics), strict impurity limits apply to glycols and polyols to exclude DEG contamination.
Applications (non-food)
DEG serves as a solvent and humectant in alkyds and polyester resins, a co-solvent in inks and coatings, a component of brake fluids and heat-transfer fluids, a processing aid in plasticizers, and an intermediate in organic synthesis. Direct use in foods or as an excipient is generally prohibited; safer alternatives such as propylene glycol are preferred where human exposure can occur.
Toxicology and human health (summary)
Absorption: rapid by oral route; dermal uptake is possible under favorable conditions.
Metabolism: oxidation via alcohol dehydrogenase yields acidic metabolites, notably diglycolic acid, which is implicated in renal tubular injury.
Clinical picture in significant exposure: central nervous system depression, metabolic acidosis, acute kidney injury, and possible hepatic involvement.
Public health context: multiple historical poisonings from adulterated medicinal syrups and fraudulent substitutions prompted stringent international restrictions and routine impurity testing.
Urgent care: any suspected exposure requires immediate medical attention; management protocols are specialized and cannot be replaced by general advice.
Regulatory framework (high-level)
DEG is not approved as a food additive or ingredient. Pharmacopoeias and regulatory standards require targeted assays for EG/DEG as impurities in at-risk excipients (for example glycerin, PEG, propylene glycol, sorbitol solution, and ethoxylated surfactants), with severe quantitative limits. In cosmetics and medical devices, DEG is treated as a technical impurity to be minimized under GMP and traceability requirements. Classification, labeling, and transport follow CLP/GHS rules for hazardous substances.
Quality, authenticity, and control
Primary risk: adulteration of pharmaceutical or food-contact polyols (for example glycerin) with DEG.
Recommended controls:
• Identity and assay: GC-FID/GC-MS or HPLC methods validated to resolve EG, DEG, TEG.
• Impurities profile: compliance with specifications limiting EG/DEG in any human-use polyol.
• General chemistry: moisture by KF, acidity/acid number, color (APHA/Hazen).
• Supply chain: vendor qualification, CoA/CoO, lot traceability, statistical sampling, and retention samples.
Best practice: never substitute glycerin or propylene glycol with DEG; enforce “gate” testing for critical raw materials.
Handling, storage, and occupational safety
Store in clean, dry, tightly closed compatible containers (stainless steel or approved drums), protected from heat and light.
Avoid contact with strong oxidizers and conditions promoting oxidation/acid formation.
Personal protective equipment: chemical-resistant gloves, splash goggles or face shield, suitable skin protection; ensure adequate ventilation or local exhaust.
Spill response: contain with inert absorbent; prevent release to drains; dispose as hazardous waste per local regulations.
Environmental considerations
DEG is generally biodegradable under aerobic conditions, but can harm aquatic organisms at elevated concentrations. Prevent releases and treat effluents appropriately. High water miscibility increases environmental mobility, making prevention paramount.
Troubleshooting (raw material and process)
Out-of-spec EG/TEG content: requalify supplier/fractionation; quarantine nonconforming lots.
Rising acidity or off-odors: indicates oxidation/contamination; check vessels, nitrogen blanketing, and lot rotation.
High water content: may drive hydrolysis or instability in sensitive systems; verify KF and dehumidification.
Slow drying in inks/coatings: review purity, viscosity, and compatibility with the resin/co-solvent system.
Conclusion
Diethylene glycol is a versatile industrial solvent and humectant that poses significant risk in any context involving human ingestion. The history of poisonings underscores the need for uncompromising regulatory compliance, targeted analytics for EG/DEG, rigorous lot traceability, and substitution with safer alternatives in sensitive applications to protect public health and corporate responsibility.
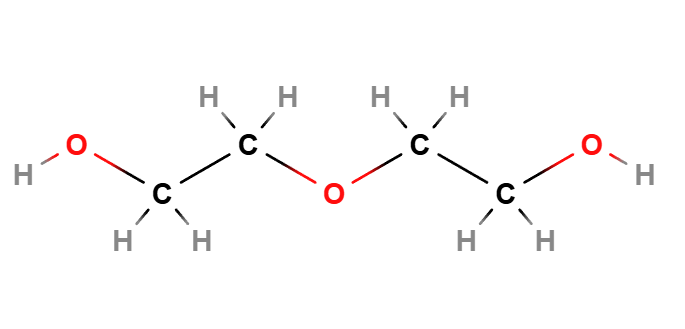 |  |
Molecular Formula C4H10O3
Molecular Weight
CAS 111-46-6
UNII 61BR964293
EC Number 203-872-2
Synonyms:
2,2'-Oxydiethanol
Diethyleneglycol
2-Hydroxyethyl ether
References__________________________________________________________________________
Hari P, Jain Y, Kabra SK. Fatal encephalopathy and renal failure caused by diethylene glycol poisoning. J Trop Pediatr. 2006 Dec;52(6):442-4. doi: 10.1093/tropej/fml040.
Abstract. Diethylene glycol poisoning results in multiorgan dysfunction and requires a high index of suspicion for diagnosis. We report 11 patients of diethylene glycol poisoning, all of whom had renal failure and severe encephalopathy. The diagnosis was established by the presence of diethylene glycol in the paracetamol elixir consumed by these children; the amount of which ranged from 2.3 to 23% w/W. Consumption of large amount of toxin resulted in severe encephalopathy and high mortality in these children.
Schep LJ, Slaughter RJ, Temple WA, Beasley DM. Diethylene glycol poisoning. Clin Toxicol (Phila). 2009 Jul;47(6):525-35. doi: 10.1080/15563650903086444. Erratum in: Clin Toxicol (Phila). 2009 Sep;47(8):840.
Abstract. Introduction: Diethylene glycol (DEG) is a clear, colorless, practically odorless, viscous, hygroscopic liquid with a sweetish taste. In addition to its use in a wide range of industrial products, it has also been involved in a number of prominent mass poisonings spanning back to 1937. Despite DEG's toxicity and associated epidemics of fatal poisonings, a comprehensive review has not been published. Methods: A summary of the literature on DEG was compiled by systematically searching OVID MEDLINE and ISI Web of Science. Further information was obtained from book chapters, relevant news reports, and web material. Aim: The aim of this review is to summarize all main aspects of DEG poisoning including epidemiology, toxicokinetics, mechanisms of toxicity, clinical features, toxicity of DEG, diagnosis, and management. Epidemiology: Most of the documented cases of DEG poisoning have been epidemics (numbering over a dozen) where DEG was substituted in pharmaceutical preparations. More often, these epidemics have occurred in developing and impoverished nations where there is limited access to intensive medical care and quality control procedures are substandard. Toxicokinetics: Following ingestion, DEG is rapidly absorbed and distributed within the body, predominantly to regions that are well perfused. Metabolism occurs principally in the liver and both the parent and the metabolite, 2-hydroxyethoxyacetic acid (HEAA), are renally eliminated rapidly. Mechanisms of toxicity: Although the mechanism of toxicity is not clearly elucidated, research suggests that the DEG metabolite, HEAA, is the major contributor to renal and neurological toxicities. Clinical features: The clinical effects of DEG poisoning can be divided into three stages: The first phase consists of gastrointestinal symptoms with evidence of inebriation and developing metabolic acidosis. If poisoning is pronounced, patients can progress to a second phase with more severe metabolic acidosis and evidence of emerging renal injury, which, in the absence of appropriate supportive care, can lead to death. If patients are stabilized, they may then enter the final phase with various delayed neuropathies and other neurological effects, sometimes fatal. TOXICITY OF DEG: Doses of DEG necessary to cause human morbidity and mortality are not well established. They are based predominantly on reports following some epidemics of mass poisonings, which may underestimate toxicity. The mean estimated fatal dose in an adult has been defined as approximately 1 mL/kg of pure DEG. Management: Initial treatment consists of appropriate airway management and attention to acid-base abnormalities. Prompt use of fomepizole or ethanol is important in preventing the formation of the toxic metabolite HEAA; hemodialysis can also be critical, and assisted ventilation may be required. Conclusions: DEG ingestion can lead to serious complications that may prove fatal. Prognosis may be improved, however, with prompt supportive care and timely use of fomepizole or ethanol.
Jamison CN, Dayton RD, Latimer B, McKinney MP, Mitchell HG, McMartin KE. Neurotoxic effects of nephrotoxic compound diethylene glycol. Clin Toxicol (Phila). 2021 Sep;59(9):810-821. doi: 10.1080/15563650.2021.1874403.
Abstract. Context: Diethylene glycol (DEG) is an organic compound found in household products but also as an adulterant in medicines by acting as a counterfeit solvent. DEG poisonings have been characterized predominately by acute kidney injury (AKI), but also by delayed neurological sequelae such as decreased reflexes or face and limb weakness. Objectives: Characterizing the neurological symptoms of DEG poisoning in a subacute animal model would create a clearer picture of overall toxicity and possibly make mechanistic connections between kidney injury and neuropathy. Methods: Male Wistar-Han rats were orally administered doses of 4 - 6 g/kg DEG every 12 or 24 h and monitored for 7 days. Urine was collected every 12 h and endpoint blood and cerebrospinal fluid (CSF) were collected for a renal plasma panel and total protein estimation, respectively. Motor function tests were conducted before and after treatment. Kidney and brain tissue was harvested for metabolic analysis. Results: Of the 43 animals treated with DEG, 11 developed AKI as confirmed by increased BUN and creatinine levels. Renal and brain DGA accumulation was markedly increased in animals that developed AKI compared to animals without AKI. The total protein content in CSF in animals with kidney injury was markedly elevated compared to control and to treated animals without AKI. Significant decreases in forelimb grip strength and decreases in locomotor and rearing activity were observed in animals with AKI compared to control and to animals without AKI. Discussion: Repeated dosing with DEG in an animal model produced nephrotoxic effects like those in studies with acute DEG administration. The decrease in motor function and increase in CSF protein were only present in animals that developed AKI. Conclusions: These studies show development of neurotoxicity in this DEG animal model and suggest that neurological symptoms are observed only when DGA accumulation and kidney injury also occur.
Tobin JD, Jamison CN, Robinson CN, McMartin KE. Variable sensitivity to diethylene glycol poisoning is related to differences in the uptake transporter for the toxic metabolite diglycolic acid. Clin Toxicol (Phila). 2023 Apr;61(4):207-211. doi: 10.1080/15563650.2022.2163659.
Abstract. Introduction/context: Poisonings with diethylene glycol are characterized by acute kidney injury and peripheral neuropathy. In animal studies on the toxicities of diethylene glycol and its metabolite diglycolic acid, remarkable differences in susceptibility to acute kidney injury were observed in identically-dosed rats. In those studies, only about 60% showed acute kidney injury, yet all rats with acute kidney injury showed marked diglycolic acid accumulation in tissues, while no diglycolic acid accumulated in rats without injury. Diglycolic acid is taken into renal cells via sodium-dependent dicarboxylate transporters. When sodium-dependent dicarboxylate transporter-1 is inhibited or knocked down in human kidney cells, diglycolic acid uptake and toxicity are reduced. We hypothesize that the variation in sensitivity to tissue diglycolic acid retention and to diethylene glycol/diglycolic acid toxicity is explained by differential expression of sodium-dependent dicarboxylate transporter-1 in rat kidneys. Methods: Using kidney tissue from previous studies, we performed rt-PCR analysis of sodium-dependent dicarboxylate transporter-1 mRNA. In those studies, Wistar-Han rats were either gavage with diethylene glycol 6 g/kg every 12 h for 7 days or with single doses of diglycolic acid 300 mg/kg. Kidney tissue was harvested after euthanasia and preserved in formalin. Tissue slices were homogenized and RNA was isolated using an RNAstorm FFPE RNA Isolation Kit. The expression of sodium-dependent dicarboxylate transporter-1 mRNA was compared between groups that showed diglycolic acid accumulation and acute renal injury with those that showed no diglycolic acid accumulation or toxicity. Results: Significantly higher expression of sodium-dependent dicarboxylate transporter-1 mRNA was present in the kidneys of rats with acute kidney injury and diglycolic acid accumulation compared to those in rats that had no diglycolic acid in their kidneys and no acute kidney injury. Discussion: The likelihood of acute kidney injury after dosing of rats with diethylene glycol or diglycolic acid is linked with an enhanced ability to take up diglycolic acid into renal cells via the sodium-dependent dicarboxylate transporter-1. The variability in diethylene glycol toxicity in humans, as reported in epidemiological studies, may also be linked with differences in tissue uptake of diglycolic acid. Conclusions: Animals with acute kidney injury after exposure to diethylene glycol or diglycolic acid had higher sodium-dependent dicarboxylate transporter-1 expression and greater diglycolic acid accumulation in renal tissues than animals without acute kidney injury.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Chemical Main substances: Last update: 2025-10-15 18:46:42 | Chemical Risk: |
