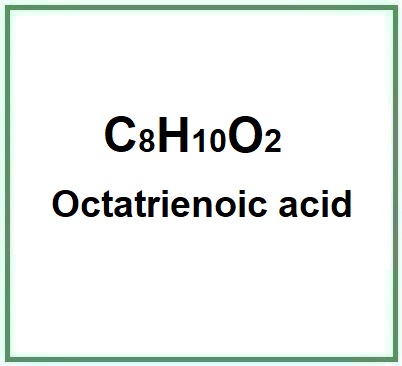
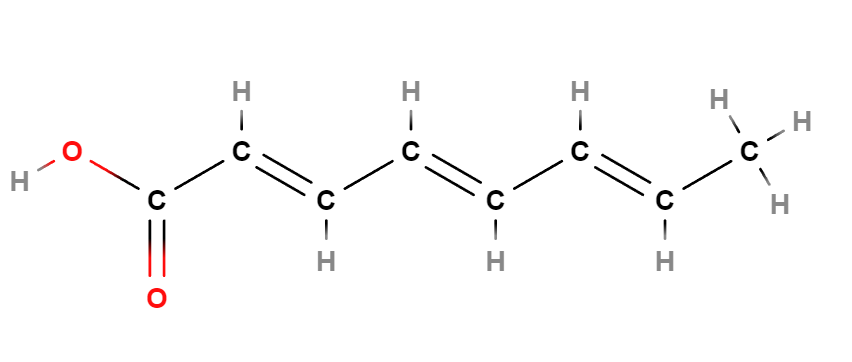
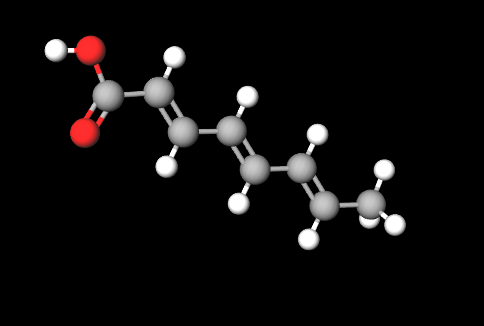
Octatrienoic Acid is a chemical compound, a fatty acid that plays a key role in many biological processes, a polyunsaturated fatty acid with three double bonds, known for its nutritional properties and potential health benefits.
The name describes the structure of the molecule:
- Octatrienoic. This indicates the presence of an eight-carbon chain with three conjugated double bonds, thus forming a triene-type structure.
- Acid. This indicates that the molecule contains a carboxylic group (-COOH), giving the molecule acidic properties.
Chemical Industrial Synthesis Process
The production of Octatrienoic Acid, also known as α-linolenic acid (ALA), an essential polyunsaturated fatty acid in the omega-3 series, involves a process that mainly includes extraction from natural sources, as this compound is abundant in nature in vegetable oils such as flaxseed oil, chia oil, hemp oil, and in certain types of nuts and seeds. Here is a detailed overview of the process.
- Selection. The first stage in the production of Octatrienoic Acid involves selecting plant sources rich in this fatty acid, such as flaxseeds, chia seeds, or nuts.
- Extraction. Oil is extracted from the seeds or nuts using cold pressing or solvent extraction. Cold pressing is preferred to preserve the nutritional properties of the oil, while solvent extraction can increase the yield of extracted oil.
- Refining. The crude oil extracted is then refined to remove impurities, undesired odors, and flavors. This may include processes such as decanting, neutralization, bleaching, and deodorization.
- Isolation. If necessary, octatrienoic acid can be isolated from the refined oil through fractionation, vacuum distillation, or chromatography processes to achieve a higher concentration of this specific fatty acid.
- Quality Control. Octatrienoic acid, whether as a component of the oil or in isolated form, undergoes quality control checks to verify purity, fatty acid concentration, and the absence of contaminants. These tests can include gas chromatography (GC) and spectroscopy.
A cosa serve e dove si usa
The function of this compound may vary depending on the context in which it is used. Fatty acids, such as octatrienoic acid, are essential for many biological processes and are precursors of bioactive molecules. In cosmetics and skin care products, they can be used for their emollient, moisturizing, and conditioning properties.
Medical
Octatrienoic acid has a protective effect against actinic keratosis (1) and against UVA- and UVB-induced damage on human keratinocytes (2).
Cosmetica - Funzioni INCI
Antioxidant agent. Ingredient that counteracts oxidative stress and prevents cell damage. Free radicals, pathological inflammatory processes, reactive nitrogen species and reactive oxygen species are responsible for the ageing process and many diseases caused by oxidation.
Applications
Nutrition. This fatty acid can play an important role in the diet, contributing to heart health and brain function due to its ability to positively influence cholesterol levels and inflammation.
Food Sources. It can be found in various food sources, including vegetable oils, nuts, and seeds, where it contributes to the diversity of essential fatty acids in the diet.
Cosmetic Applications. In the cosmetic industry, octatrienoic acid may be used in skincare formulations for its moisturizing, antioxidant, and regenerative properties.
Skin Benefits. Due to its polyunsaturated structure, it can help strengthen the skin barrier, improve skin elasticity, and reduce signs of aging.
Research and Development. Ongoing research continues to explore the potential of this fatty acid in areas such as chronic disease prevention, optimal nutrition, and therapeutic applications in skincare.
Molecular Formula C8H10O2
Molecular Weight 138.16 g/mol
CAS 5205-32-3
UNII 702XZO95X4
EC Number 622-483-2
DTXSID20242869
Nikkaji J1.100.542J J2.629.767B
Synonyms:
α-Linolenic Acid
Bibliografia_____________________________________________________________________
(1) Pinto D, Trink A, Giuliani G, Rinaldi F. Protective effects of sunscreen (50+) and octatrienoic acid 0.1% in actinic keratosis and UV damages. J Investig Med. 2022 Jan;70(1):92-98. doi: 10.1136/jim-2021-001972.
(2) Flori E, Mastrofrancesco A, Kovacs D, Bellei B, Briganti S, Maresca V, Cardinali G, Picardo M. The activation of PPARγ by 2,4,6-Octatrienoic acid protects human keratinocytes from UVR-induced damages. Sci Rep. 2017 Aug 23;7(1):9241. doi: 10.1038/s41598-017-09578-3.
Abstract. Increasing attention is addressed to identify products able to enhance skin photoprotection and to prevent skin carcinogenesis. Several studies have demonstrated that the α-melanocyte stimulating hormone (αMSH), acting on a functional MC1R, provides a photoprotective effect by inducing pigmentation, antioxidants and DNA repair. We discovered a link between αMSH and the nuclear receptor Peroxisome Proliferator-Activated Receptor-γ (PPARγ), suggesting that some of the αMSH protective effects may be dependent on PPARγ transcriptional activity. Moreover, we demonstrated that the activation of PPARγ by the parrodiene 2,4,6-octatrienoic acid (Octa) induces melanogenesis and antioxidant defence in human melanocytes and counteracts senescence-like phenotype in human fibroblasts. In this study, we demonstrate that the activation of PPARγ by Octa exerts a protective effect against UVA- and UVB-induced damage on normal human keratinocytes (NHKs), the major target cells of UV radiation. Octa promotes the antioxidant defence, augments DNA repair and reduces the induction of proteins involved in UV-induced DNA damage response. Our results contribute to deepen the analysis of the αMSH/PPARγ connection and suggest perspectives for the development of new molecules and formulations able to prevent cutaneous UV damage by acting on the different skin cell populations through PPARγ activation.
(3) Flori E, Mastrofrancesco A, Kovacs D, Ramot Y, Briganti S, Bellei B, Paus R, Picardo M. 2,4,6-Octatrienoic acid is a novel promoter of melanogenesis and antioxidant defence in normal human melanocytes via PPAR-γ activation. Pigment Cell Melanoma Res. 2011 Aug;24(4):618-30. doi: 10.1111/j.1755-148X.2011.00887.x. Epub 2011 Aug 11. PMID: 21762468.
![]() Octatrienoic Acid
Octatrienoic Acid