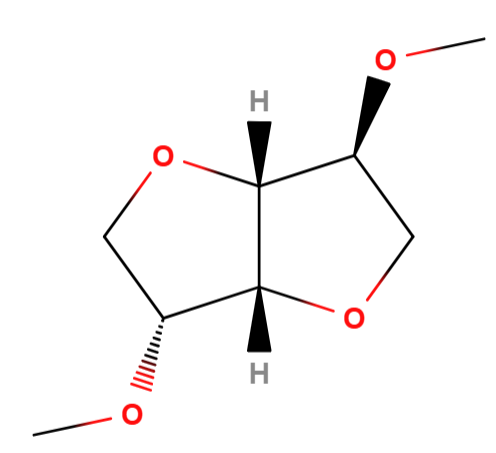
Dimethyl Isosorbide is a chemical compound, green bio-based solvent. Dimethyl Isosorbide is a derivative of isosorbide, a biobased compound obtained from the dehydration of glucose. It is a non-toxic and biodegradable solvent used in cosmetics and pharmaceuticals. Due to its ability to enhance the solubility of various active ingredients and penetrate the skin, it is often employed to increase the effectiveness of skincare products.
The name describes the structure of the molecule
- "Dimethyl" indicates that there are two methyl groups (-CH3) attached to the molecule.
- "Isosorbide" is a compound derived from sorbitol, an alcohol sugar used in various fields, including medicine and cosmetics, for its solubilizing properties and as a vehicle for other ingredients.
Description of raw materials used in production
- Isosorbide - A diol derived from glucose.
- Methanol - Used to methylate isosorbide.
Step-by-step summary of industrial chemical synthesis process
- Preparation - Isosorbide and methanol are prepared under controlled conditions.
- Methylation Reaction - Isosorbide reacts with methanol in the presence of an acid catalyst. This reaction yields Dimethyl Isosorbide as the product.
- Purification - The resultant product is purified via distillation to remove impurities and other compounds.
It appears as a colorless liquid.

What it is for and where
Pharmaceuticals
Dimethyl Isosorbide is a solvent and drug delivery agent, enhances the solubility of active ingredients and increases their penetration through the skin (1).
Cosmetics
Solvent. It is the substance for dissolving or dispersing surfactants, oils, dyes, flavourings, bactericidal preservatives in solution.In fact, it dissolves other components present in a cosmetic formulation. Solvents are generally liquid (aqueous and non-aqueous). (2).
Viscosity control agent. It controls and adapts, Increasing or decreasing, viscosity to the required level for optimal chemical and physical stability of the product and dosage in gels, suspensions, emulsions, solutions.
Commercial applications
Cosmetics and Skincare. Used as a carrier to enhance the absorption of active ingredients into the skin, making products more effective.
Hair Care Products. Employed in hair care formulations to enhance the penetration of active ingredients into the scalp.
Fragrance Formulations. Acts as a solvent in certain fragrance formulations.
Sun Care Products. Can be used in sun care products to improve the absorption of UV filters (3).
Medical Applications
Topical Products. Used in medicinal creams, lotions, and gels to enhance the delivery of therapeutic active ingredients to the skin or scalp.
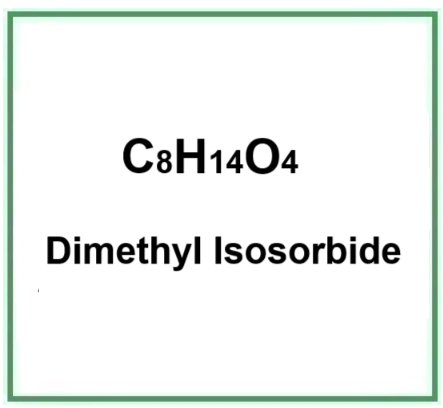
- Molecular Formula C8H14O4
- Molecular Weight 174.19 g/mol
- CAS 5306-85-4
- UNII SA6A6V432S
- EC Number 226-159-8
- DTXSID201019399
- Nikkaji J208.650F
Synonyms:
- 2,5-dimethylisosorbide
- Isosorbide dimethyl ether
References_____________________________________________________________________
(1) Zia H, Ma JK, O'Donnell JP, Luzzi LA. Cosolvency of dimethyl isosorbide for steroid solubility. Pharm Res. 1991 Apr;8(4):502-4. doi: 10.1023/a:1015807413141.
Abstract. Dimethyl isosorbide (DMI), which is currently under investigation for its potential use as a pharmaceutical vehicle and drug permeation enhancer, is a water-miscible liquid with relatively low viscosity. The solubilization behavior of DMI as a cosolvent for nonpolar drugs was characterized via dielectric constant measurements of binary solvent systems containing DMI and either water, propylene glycol (PG), or polyethylene glycol (PEG). Evidence from the dielectric constant profiles and NMR studies suggest that DMI undergoes complexation with water and PG, but not with PEG, through hydrogen bonding interactions. The solvent complexation exhibited a major effect on the solubilities of prednisone, dexamethasone, and prednisolone in the mixed solvent systems. Maximum solubility of each drug was found to occur near a DMI/water or DMI/PG concentration ratio of 1:2. In the DMI-PEG mixed system, while there is no apparent interaction between DMI and PEG molecules, the solubility of prednisone was found to increase with decreasing dielectric constant.
(2) Su M, Huang X, Lei C, Jin J. Nickel-Catalyzed Reductive Cross-Coupling of Aryl Bromides with Vinyl Acetate in Dimethyl Isosorbide as a Sustainable Solvent. Org Lett. 2022 Jan 14;24(1):354-358. doi: 10.1021/acs.orglett.1c04018. Epub 2021 Dec 28. PMID: 34962407.
Abstract. A nickel-catalyzed reductive cross-coupling has been achieved using (hetero)aryl bromides and vinyl acetate as the coupling partners. This mild, applicable method provides a reliable access to a variety of vinyl arenes, heteroarenes, and benzoheterocycles, which should expand the chemical space of precursors to fine chemicals and polymers. Importantly, a sustainable solvent, dimethyl isosorbide, is used, making this protocol more attractive from the point of view of green chemistry.
(3) Piccinino D, Capecchi E, Trifero V, Tomaino E, Marconi C, Del Giudice A, Galantini L, Poponi S, Ruggieri A, Saladino R. Lignin Nanoparticles as Sustainable Photoprotective Carriers for Sunscreen Filters. ACS Omega. 2022 Oct 10;7(42):37070-37077. doi: 10.1021/acsomega.2c02133.
Abstract. Sunscreen filters may be degraded after prolonged UV exposure with loss of their shielding property and generation of harmful radical species. They are contained in cosmetic formulations in high concentrations, so the improvement of photostability is of relevance for safety concerns. We report here that lignin nanoparticles are sustainable carriers and photostabilizers of two common UV chemical filters, namely, avobenzone and octyl methoxycinnamate. These compounds have been encapsulated by nanoprecipitation into kraft lignin nanoparticles using eco-certified dimethyl isosorbide as a primary solvent and deionized water as an antisolvent. After the encapsulation, both compounds significantly prolonged the half-life stability against UV irradiation. The stabilizing properties of lignin nanoparticles were further improved by coencapsulation of avobenzone and octyl methoxycinnamate with hydroxytyrosol, a natural phenol with antioxidant activity recovered from olive oil wastes and characterized by skin regenerative properties. © 2022 The Authors. Published by American Chemical Society.
![]() Dimethyl Isosorbide
Dimethyl Isosorbide