Potassium hydrogen carbonate (Potassium bicarbonate) is a chemical compound, potassium salt of carbonic acid, produced by the passage of carbon dioxide through an aqueous solution of potassium carbonate.
The name describes the structure of the molecule:
- Potassium refers to the presence of potassium ions (K+) in the compound.
- Bicarbonate refers to the bicarbonate ion (HCO3-), which consists of one hydrogen atom, one carbon atom and three oxygen atoms. The term 'bi' in bicarbonate indicates the presence of two different types of oxygens: those of the carbonate ion (CO32-) and an additional hydroxyl group (OH-).
The synthesis process takes place in different steps:
- Preparation of potassium carbonate or potassium hydroxide solution. Potassium carbonate or potassium hydroxide is dissolved in water.
- Carbonation. Carbon dioxide is made to flow through the solution. CO2 reacts with the potassium carbonate or potassium hydroxide to form potassium bicarbonate.
Industrially It appears in the form of a white powder.

What it is for and where
Medical
Potassium hydrogen carbonate is used in medicine as an antacid and in the treatment of gastroesophageal reflux.
- Antacid. Potassium bicarbonate is able to neutralise gastric juices, making it an effective antacid. It can be used to treat conditions such as acid indigestion and heartburn.
- Electrolyte replenisher. Can be used to replenish potassium in the body, which is essential for different bodily functions, including the act of nerve and muscle cells, heart function and the maintenance of fluid balance.
- Potassium supplement. It is used as a potassium supplement in situations where people are at risk of or suffer from hypokalaemia (low blood potassium levels). This can occur due to conditions such as chronic diarrhoea, vomiting, malabsorption syndromes or certain medications.
- Urinary alkaliniser. Can be used to make urine more alkaline (i.e. less acidic). It may be useful in treating certain types of urinary tract infections and may help prevent the formation of certain types of kidney stones.
In dietary supplements, potassium bicarbonate is used as a source of potassium, an essential mineral for muscle and nerve function. It contributes to the maintenance of electrolyte balance. It acts as a buffering agent, helping regulate acid–base balance. It can support normal cardiac function. It is used to help counteract mild metabolic acidosis. It may contribute to blood pressure control. It can also serve a technological role as a pH regulator.
Studies
A new extended-release formulation of potassium citrate and potassium bicarbonate, ADV7103, has been shown to improve metabolic control, palatability, and gastrointestinal safety in patients with distal renal tubular acidosis (dRTA) compared with standard of care (SoC) treatments. The present work evaluates the safety and efficacy of ADV7103 during 24 months (1).
Potassium hydrogen carbonate, in conjunction with whey protein, may improve oxidative metabolism due to prolonged bed rest (2).
Potassium hydrogen carbonate (PB), calcium chelate (CCh), and sodium silicate (SSi) have been extensively used as antifungal generally recognized as safe (GRAS) compounds against plant pathogenic fungi (3).
Cosmetics
Bulking agent. It regulates the water content, dilutes other solids, can increase the volume of a product for better flow, acts as a buffer against organic acids, helps to keep the pH of the mixture within a certain level.

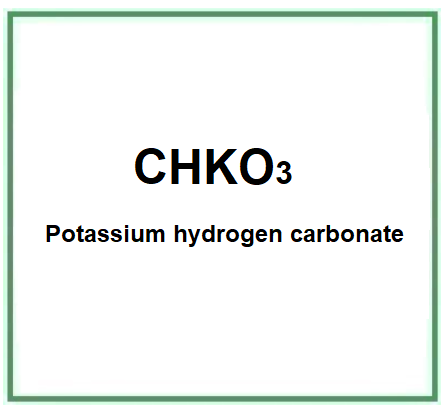
- Molecular Formula: KHCO3 o CHKO3
- Molecular Weight: 100.115 g/mol
- CAS: 298-14-6
- UNII HM5Z15LEBN
- EC Number: 241-378-9 206-059-0
- DSSTox Substance ID: DTXSID0021177
- MDL number MFCD00011402
- PubChem Substance ID 329752450
- NACRES: NB.24
- Beilstein/REAXYS Number: 4535309
Synonims:
- Potassium hydrogen carbonate
- Carbonic acid, potassium salt
- Potassium hydrogencarbonate
- Potassiumhydrogencarbonate
- Potassium acid carbonate
- Potassium bicarbonate
References________________________________________________________________
(1) Bertholet-Thomas A, Guittet C, Manso-Silván MA, Joukoff S, Navas-Serrano V, Baudouin V, Cailliez M, Di Maio M, Gillion-Boyer O, Golubovic E, Harambat J, Knebelmann B, Nobili F, Novo R, Podracka L, Roussey-Kesler G, Granier LA. Safety, efficacy, and acceptability of ADV7103 during 24 months of treatment: an open-label study in pediatric and adult patients with distal renal tubular acidosis. Pediatr Nephrol. 2021 Feb 26. doi: 10.1007/s00467-020-04873-0.
Abstract. Background: A new prolonged-release formulation of potassium citrate and potassium bicarbonate, ADV7103, has been shown to improve metabolic control, palatability, and gastrointestinal safety in patients with distal renal tubular acidosis (dRTA) when compared to standard of care (SoC) treatments. The present work evaluates safety and efficacy of ADV7103 during 24 months. Methods: Thirty pediatric and adult patients were included in an open-label extension study after a phase II/III trial. Safety and tolerability were assessed. Plasma bicarbonate and potassium levels, as well as urine parameters, were evaluated over time. Acceptability, adherence, and quality of life were also assessed. The evolution of clinical consequences of dRTA in the cohort was explored. Results: There were 104 adverse events (AEs) reported, but only 9 gastrointestinal events observed in five patients (17%) were considered to be related to ADV7103 treatment. There were no AEs leading to treatment discontinuation. Plasma bicarbonate and potassium levels were in the normal ranges at the different visits, respectively, in 69-86% and 83-93% of patients. Overall adherence rates were ≥ 75% throughout the whole study in 79% patients. An average improvement of quality of life of 89% was reported at 24 months of study. Conclusions: Common AEs concerned metabolism and gastrointestinal disorders; the former being related to the disease. Less than half of the gastrointestinal AEs were related to ADV7103 treatment and they were mostly mild in severity. Metabolic parameters were maintained in the normal ranges in most patients. Patient satisfaction was high and adherence to treatment was good and remained stable.
(2) Bosutti A, Mulder E, Zange J, Bühlmeier J, Ganse B, Degens H. Effects of 21 days of bed rest and whey protein supplementation on plantar flexor muscle fatigue resistance during repeated shortening contractions. Eur J Appl Physiol. 2020 May;120(5):969-983. doi: 10.1007/s00421-020-04333-5.
Abstract. Purpose: Space flight and bed rest (BR) lead to a rapid decline in exercise capacity. Whey protein plus potassium bicarbonate diet-supplementation (NUTR) could attenuate this effect by improving oxidative metabolism. We evaluated the impact of 21-day BR and NUTR on fatigue resistance of plantar flexor muscles (PF) during repeated shortening contractions, and whether any change was related to altered energy metabolism and muscle oxygenation. Methods: Ten healthy men received a standardized isocaloric diet with (n = 5) or without (n = 5) NUTR. Eight bouts of 24 concentric plantar flexions (30 s each bout) with 20 s rest between bouts were employed. PF muscle size was assessed by means of peripheral quantitative computed tomography. PF muscle volume was assessed with magnetic resonance imaging. PF muscle force, contraction velocity, power and surface electromyogram signals were recorded during each contraction, as well as energy metabolism (31P nuclear magnetic resonance spectroscopy) and oxygenation (near-infrared spectroscopy). Cardiopulmonary parameters were measured during an incremental cycle exercise test. Results: BR caused 10-15% loss of PF volume that was partly recovered 3 days after re-ambulation, as a consequence of fluid redistribution. Unexpectedly, PF fatigue resistance was not affected by BR or NUTR. BR induced a shift in muscle metabolism toward glycolysis and some signs of impaired muscle oxygen extraction. NUTR did not attenuate the BR-induced-shift in energy metabolism. Conclusions: Twenty-one days' BR did not impair PF fatigue resistance, but the shift to glycolytic metabolism and indications of impaired oxygen extraction may be early signs of developing reduced muscle fatigue resistance.
(3) Youssef K, Roberto SR, de Oliveira AG. Ultra-Structural Alterations in Botrytis cinerea-The Causal Agent of Gray Mold-Treated with Salt Solutions. Biomolecules. 2019 Oct 8;9(10):582. doi: 10.3390/biom9100582.
![]() Potassium hydrogen carbonate
Potassium hydrogen carbonate