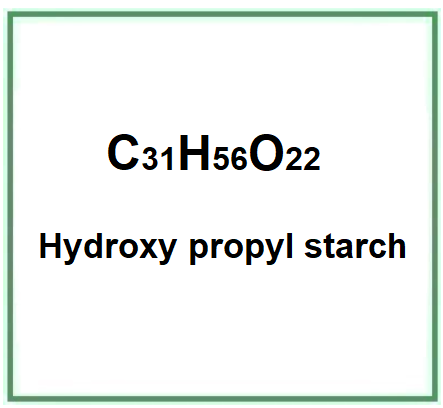
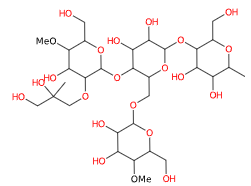
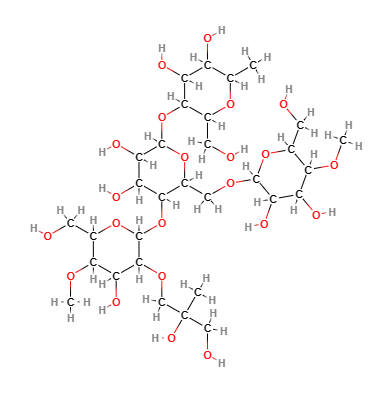
E1440 (Hydroxy propyl starch) is a modified starch obtained by etherification of food starch (maize, rice) with propylene oxide (10%) and sodium hydroxide (1%).
It appears as a white powder or white granules.

What it is used for and where
Food
Ingredient included in the list of European food additives as E1440 as a stabiliser, thickener and for its film-forming properties (1).
Safety
The EFSA Panel on Food Additives and Nutrient Sources Added to Food considers that there is no safety concern for the use of modified starches as food additives at the uses and use levels declared for the general population and that a numerical ADI is not necessary (2).
Other uses
Textile and paper industry.
- Molecular Formula C31H56O22
- Molecular Weight 780.8
- CAS 9049-76-7
- UNII 9M44R3409A
- EC Number 618-565-2
References_____________________________________________________________________
(1) Arvanitoyannis, I., Nakayama, A. and Aiba, S.I., 1998. Edible films made from hydroxypropyl starch and gelatin and plasticized by polyols and water. Carbohydrate polymers, 36(2-3), pp.105-119.
Abstract. Two methods, known as the low and the high temperature methods, which consist of casting aqueous solutions of hydroxypropyl starch and gelatin at 20 and 60°C, respectively, were employed for film preparation. The physical (thermal, mechanical and gas/water permeation) properties of these composite films, plasticized with water or polyols, were studied. An increase in the total plasticizer content resulted in a considerable decrease in elasticity modulus and tensile strength (up to 60% of the original values when 25% plasticizer was added), whereas the percentage elongation increased (up to 200% compared to the original values). The low temperature method led to the development of higher percentage renaturation (crystallinity) of gelatin which resulted in a decrease, by one or two magnitude orders, of CO2 and O2 permeability in the hydroxypropyl starch/gelatin blends. An increase in the total plasticizer content (water, polyols) of these blends was found to be proportional to an increase in their gas permeability.
(2) EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), Mortensen, A., Aguilar, F., Crebelli, R., Di Domenico, A., Dusemund, B., Frutos, M.J., Galtier, P., Gott, D., Gundert‐Remy, U. and Lambré, C., 2017. Re‐evaluation of oxidised starch (E 1404), monostarch phosphate (E 1410), distarch phosphate (E 1412), phosphated distarch phosphate (E 1413), acetylated distarch phosphate (E 1414), acetylated starch (E 1420), acetylated distarch adipate (E 1422), hydroxypropyl starch (E 1440), hydroxypropyl distarch phosphate (E 1442), starch sodium octenyl succinate (E 1450), acetylated oxidised starch (E 1451) and starch aluminium octenyl succinate (E 1452) as food additives. EFSA Journal, 15(10), p.e04911.
Abstract. Following a request from the European Commission, the EFSA Panel on Food Additives and Nutrient sources added to Food (ANS) was asked to deliver a scientific opinion on the re-evaluation of 12 modified starches (E 1404, E 1410, E 1412, E 1413, E 1414, E 1420, E 1422, E 1440, E 1442, E 1450, E 1451 and E 1452) authorised as food additives in the EU in accordance with Regulation (EC) No 1333/2008 and previously evaluated by JECFA and the SCF. Both committees allocated an acceptable daily intake (ADI) ‘not specified’. In humans, modified starches are not absorbed intact but significantly hydrolysed by intestinal enzymes and then fermented by the intestinal microbiota. Using the read-across approach, the Panel considered that adequate data on short- and long-term toxicity and carcinogenicity, and reproductive toxicity are available. Based on in silico analyses, modified starches are considered not to be of genotoxic concern. No treatment-related effects relevant for human risk assessment were observed in rats fed very high levels of modified starches (up to 31,000 mg/kg body weight (bw) per day). Modified starches (e.g. E 1450) were well tolerated in humans up to a single dose of 25,000 mg/person. Following the conceptual framework for the risk assessment of certain food additives, the Panel concluded that there is no safety concern for the use of modified starches as food additives at the reported uses and use levels for the general population and that there is no need for a numerical ADI. The combined exposure to E 1404–E 1451 at the 95th percentile of the refined (brand-loyal) exposure assessment scenario for the general population was up to 3,053 mg/kg bw per day. Exposure to E 1452 for food supplement consumers only at the 95th percentile was up to 22.1 mg/kg bw per day.
![]() E1440
E1440