E1207 (Anionic methacrylate copolymer) is a chemical compound produced in aqueous solution through a polymerisation process with methyl methacrylate, methacrylic acid and methyl acrylate. The free radical initiators are polysorbate 80 and sodium lauryl sulphate. The latter compound is not an authorised food additive. Simethicone provides foam reduction.
It appears in the form of a white water-soluble powder.

What it is used for and where
Food
Ingredient listed in the European food additives list as E1207 as a coating agent in solid food supplements (capsules, tablets, pills, pellets and powders).
Medical
Anionic methacrylate copolymer is used in microcapsules as drug release at a very high zero-order rate
Safety
EFSA's Scientific Panel on Food Additives and Nutrient Sources Added to Food considered that the use of AMC in solid food supplements at the proposed use and use levels does not raise safety concerns. The Panel was unable to assess the safety of anionic methacrylate copolymer for use in solid foods for special medical purposes (1).
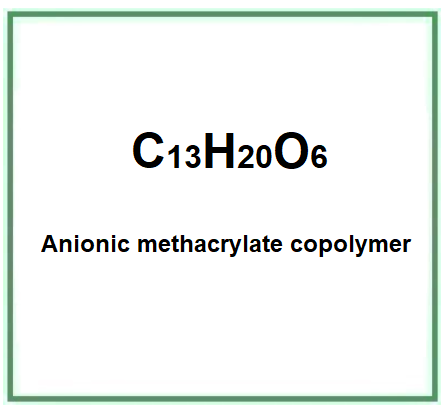
- Molecular Formula C13H20O6
- Molecular Weight 272.29
- CAS 26936-24-3
- UNII 99Q3C7L77T
- EC Number
References_____________________________________________________________________
(1) EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), 2010. Scientific Opinion on the safety of anionic methacrylate copolymer for the proposed uses as a food additive. EFSA Journal, 8(7), p.1656.
Abstract. The Panel on Food Additives and Nutrient Sources added to Food provides a scientific opinion on the use of anionicmethacrylate copolymer (AMC, a 30% dispersion of the dry copolymer in water) as a coating agent for solid foodsupplements and solid foods for special medical purposes (FSMPs). The dispersion contains 0.3% sodium laurylsulphate, which is not an authorised food additive. The opinion does not include a safety evaluation of thissubstance. From studies on toxicokinetics, acute and subchronic oral toxicity, genotoxicity, and developmentaltoxicity it is concluded that AMC is essentially not absorbed and that if any very low amounts of material wereabsorbed such material would not be retained in the tissues. No data on reproductive toxicity, chronic toxicity andcarcinogenicity are provided. In the absence of such data, chronic effects in the gastrointestinal tract following oraladministration cannot be excluded. Therefore, the Panel considers that an ADI should not be established, and that amargin of safety (MOS) approach is appropriate. Data from in vitro Ames and mammalian cell mutation assays andan in vivo micronucleus assay do not raise concern with respect to genotoxicity. A subchronic toxicity study and adevelopmental toxicity study in the rat provided NOAELs of respectively 1500 mg/kg bw/day (highest dose tested)and 1000 mg/kg bw/day (one dose tested). The anticipated exposure to AMC from both its use in food supplementsand in pharmaceuticals is 23.4 mg/kg bw/day for high consumer adults and 16 mg/kg bw/day for children. From the NOAELs, a coating level of 100 mg/tablet AMC and a combined exposure (food supplements and pharmaceuticals), MOS values between 43 to 64 for adults and 63 to 94 for children were calculated. The Panel considers these MOSsufficient given the lack of absorption and that the exposure estimates are based on worst case assumptions. The Panel concludes that the use of AMC in solid food supplements at the proposed use and use levels is not of safetyconcern. The Panel could not assess the safety of anionic methacrylate copolymer for uses in solid foods for specialmedical purposes.
![]() E1207
E1207