![]() E224
E224
Rating : 5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Cons:
Possible specific allergy (1)10 pts from FCS777
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about E224 Review Consensus 10 by FCS777 (5436 pt) | 2025-Oct-12 10:34 |
| Read the full Tiiip | (Send your comment) |
E224 (Potassium metabisulphite) is a chemical compound that belongs to the sulphite group, sulphur-based components that release sulphur dioxide SO2, an active preservative compound, a white granular powder.

E224 is a food additive known as potassium metabisulfite.
The name describes the structure of the molecule:
- The letter E followed by a number is a coding system used in the European Union to identify food additives approved for use in food. These additives are tested for safety and are listed with an "E" code followed by a unique number.
- 224 is the specific identifier for potassium metabisulfite. This compound is used as a preservative and antimicrobial agent in various food products. The E200 series (E200-E297) lists preservatives and a few decolorizers, mostly chemical.
Potassium Metabisulfite
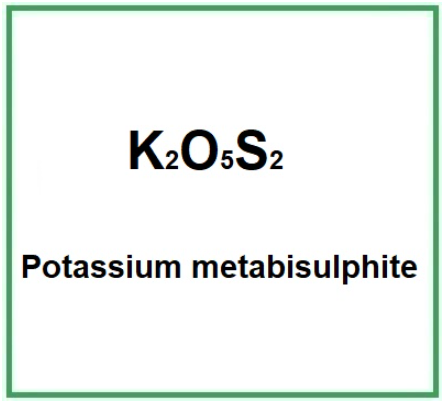
Synonyms / codes: potassium pyrosulfite, potassium disulfite, K₂S₂O₅, food additive E224.
Not to be confused with sodium metabisulfite (E223).
Definition
Acid salt of sulfurous acid used as a preservative/antimicrobial, antioxidant, anti-browning agent, and mild bleaching agent. In water it hydrolyzes to bisulfite and releases sulfur dioxide (SO₂), which is primarily responsible for its technological action.
Caloric value
0 kcal per 100 g (inorganic salt; no metabolizable energy).
Composition and essential chemistry
Formula: K₂S₂O₅ (molar mass ≈ 222.33 g/mol).
In water: K₂S₂O₅ + H₂O ⇌ 2 KHSO₃ (potassium bisulfite) ⇌ SO₂ (aq) + HSO₃⁻ + H⁺.
pH-dependent speciation: antimicrobial efficacy rises at lower pH because the fraction of molecular SO₂ increases (the membrane-permeable form).
Typical fraction of molecular SO₂ within the “free SO₂” pool: pH 3.0 ≈ 6–7%; pH 3.2 ≈ ~4%; pH 3.4 ≈ 2.5–3%; pH 3.6 ≈ 1.5–2%.
Physicochemical properties (indicative)
Appearance: white crystalline powder; pungent sulfurous odor.
Solubility: high in water; solutions are acidic (pH ~3.5–5).
Stability: sensitive to air/moisture/heat (oxidizes to sulfate and liberates SO₂).
Thermal decomposition: above ~150–170 °C with SO₂ release.
Compatibility: may reduce/bleach some colorants and is catalytically oxidized in the presence of metals—use appropriate equipment and chelators when needed.
Mechanisms of action
Antimicrobial: molecular SO₂ crosses membranes and inhibits key enzymes in yeasts and bacteria.
Antioxidant: reduces quinones and other oxidized species (protects against enzymatic browning and oxidation in juices, wine, cut fruit).
Carbonyl binding: forms adducts with acetaldehyde and other carbonyls (useful in winemaking to “bind” off-aldehydes).
Anti-browning / anti-Maillard: inhibits polyphenol oxidase and scavenges reactive intermediates.
Manufacture (brief)
React SO₂ with potassium carbonate/hydroxide to form bisulfite, then concentrate/crystallize to metabisulfite. Dry, mill, and pack in barrier containers.
Primary applications
Winemaking (enology): must sanitation and oxidative protection; microflora control.
Practical targets are expressed as free SO₂ and molecular SO₂ (e.g., ≈ 0.5 mg/L molecular SO₂ for many white wines; the required free SO₂ depends strongly on pH).
Additions pre-fermentation, at rackings, and pre-bottling; avoid excess that can mask aroma.
Brewing: water treatment (neutralizing chlorine/chloramines) and occasional antioxidant use; common homebrew practice is a Campden tablet for ~20 L water (adjust to actual chloramine levels).
Juices, purées, cut/dried fruit: anti-browning and antimicrobial action (use within category limits).
Cosmetics / OTC: antioxidant and secondary preservative (INCI: Potassium Metabisulfite) in certain aqueous solutions; more common in rinse-off products.
Indicative dosing (validate in your matrix and per local law)
Wine/must: dose to achieve target free and molecular SO₂; compute using tables/tools that account for pH and temperature.
Water treatment (homebrewing): ~1 Campden tablet (often ~0.44 g K₂S₂O₅) per 20–25 L for low chloramine loads; verify residuals and adjust.
Fruit and juices: follow legal maximums and good manufacturing practice; consider alternatives (ascorbic/citric acid + pasteurization) as appropriate.
Regulatory and allergen aspects
E224 permitted in many food categories with maximum limits per product type; in wine, limits are style-dependent (vary with sugar, type, etc.).
Allergen labeling (EU): mandatory declaration “contains sulfites” when total SO₂ ≥ 10 mg/kg or 10 mg/L (as SO₂).
Cosmetics (EU): allowed as antioxidant/preservative under GMP; consider pH and potential sensitization in hyper-reactive individuals.
Safety and risk management
Sulfite sensitivity: may trigger asthma/bronchospasm or intolerance in susceptible individuals (especially asthmatics). Minimize exposure and avoid in at-risk populations.
Occupational exposure: SO₂ vapors pose risks—work in ventilated areas; use PPE (respirators/eye/skin protection).
Equipment compatibility: avoid catalytic metals; prefer suitable stainless steels or compatible polymers.
Quality, control, and storage
Typical spec: high K₂S₂O₅ assay, very low heavy metals, impurities within standards.
Storage: dry, airtight, protected from light/heat; reseal promptly to avoid moisture uptake and oxidation (loss of strength).
Shelf life: 12–24 months unopened under proper storage; re-check assay for critical dosing after long storage.
Practical notes (enology/processing)
Antimicrobial impact is pH-driven: at pH 3.1–3.3 less free SO₂ is needed to reach a given molecular SO₂ than at pH 3.5–3.6.
SO₂ becomes bound (e.g., to acetaldehyde), lowering the measurable free fraction: monitor free and total SO₂ and adjust across rackings.
Combine with good oxygen management (inert gas, full vessels, low temperatures). If using ascorbic acid, do so only with adequate free SO₂ to avoid pro-oxidant cascades.
Conclusion
Potassium metabisulfite (E224) is a versatile and effective tool for oxidation control and microbial management in wine, juices, and fruit processing, also useful in brewing water treatment and certain cosmetic formulas. Informed use requires attention to pH, dose, and SO₂ speciation, strict adherence to regulatory limits, and rigorous safety/labeling practices to protect consumers sensitive to sulfites.
The sulphite group includes:
| Sulphur dioxide | E220 | SO2 |
| Sodium sulphite | E221 | Na2SO3 |
| Sodium hydrogen sulphite | E222 | NaHO3S |
| Sodium metabisulphite | E223 | Na2O5S2 |
| Potassium metabisulphite | E224 | K2O5S2 |
| Calcium sulphite | E226 | CaSO3 |
| Calcium hydrogen sulphite | E227 | CaH2O6S2 |
| Potassium hydrogen sulphite | E228 | KHSO3 |
What it is used for and where
It is used in the food sector as preservative and antioxidant and is labeled in Europe with the number E224 in food additives.
Can give allergy.
Most significant studies:
It is added to winesas preservative and appears on the label as "Sulfites", in vegetables and dried fruit and dried fruit, mushrooms and in many food products to increase the storage time (1).
It is also used to obtain better sensory qualities to foods (2).
In addition to essential oils, potassium metabisulfite (KMS) may be useful as a repellent for Drosophila suzukii, a globally invasive pest of soft-skinned fruit (3).
Safety
Symptoms related to sulphite sensitivity can be of varying nature and importance. The most common are headache and generalised itching or swelling, but cases of nausea, bronchoconstriction, diarrhoea, hypotension and shock have also occurred (4).
EFSA's Scientific Panel on Food Additives and Flavourings assessed the risk for toxic elements in sulphur dioxide (E 220-228), based on data submitted by stakeholders, and concluded that the EU specification maximum limits for arsenic, lead and mercury should be lowered and a maximum limit for cadmium should be introduced (5).
The Cosmetic Ingredient Review Expert Panel concluded that Sodium Sulfite, Potassium Sulfite, Ammonium Sulfite, Sodium Bisulfite, Ammonium Bisulfite, Sodium Metabisulfite, and Potassium Metabisulfite are safe as used in cosmetic formulations (6).
Molecular Formula K2S2O5 or K2O5S2
Molecular Weight 222.312 g/mol
UNII 65OE787Q7W
CAS 4429-42-9 16731-55-8
EC Number 240-795-3
Synonyms :
- Potassium metabisulfite
- Dipotassium disulfite
- Dipotassium pyrosulfite
- Dipotassium disulphite
- Dipotassium metabisulfite
- E224
- Potassium disulfite
- Potassium pyrosulfite
- Disulfurous acid, potassium salt (1:2)
- Potassium metabisulfite (NF)
References_________________________________________________________________
(1) Diamante LM, Bai X, Busch J. Fruit Leathers: Method of Preparation and Effect of Different Conditions on Qualities. Int J Food Sci. 2014;2014:139890. doi: 10.1155/2014/139890. Epub 2014 May 4. Review.
Abstract. Fruit leathers are dehydrated fruit products which are eaten as snacks or desserts. They are flexible sheets that have a concentrated fruit flavor and nutritional aspects. Most fruit leathers are prepared by mixing fruit puree and other additives like sugar, pectin, acid, glucose syrup, color, and potassium metabisulphite and then dehydrating them under specific conditions. Various drying systems including combined convective and far-infrared drying, hot air drying, microwave drying, solar drying, and sun drying have been used to make fruit leathers. Most fruit leathers are dried at 30 to 80°C for up to 24 hours until the target final moisture content (12-20%) has been reached. Research about fruit leathers began in the 1970s. This work has reviewed published papers on fruit leathers in order to summarize useful information about fruit leathers on methods of preparation, effects of drying condition, and effects of packaging and storage, which will be useful to many in the food industry and consumers who are health-conscious.
Kumar A, Singh M, Singh G. Effect of different pretreatments on the quality of mushrooms during solar drying. J Food Sci Technol. 2013 Feb;50(1):165-70. doi: 10.1007/s13197-011-0320-5.
Abstract. Freshly harvested mushrooms are highly perishable because of high moisture content metabolism and susceptible to enzymatic browning. Mushroom is a fungal fruiting body which is cultivated throughout the world. Effect on quality of dried mushrooms was studied for various chemical pretreatments viz. 1.0% potassium metabisulphite, 0.5% citric acid, 0.5% potassium metabisulphite + 0.2% citric acid, control and low cost drying methods viz. domestic solar dryer, medium size solar dryer and open sun drying. It was observed that application of 1% potassium metabisulphite treatment prior to drying using medium size solar dryer gave best quality dried mushrooms with results in accordance with statistical analysis. The drying time and final moisture content was also comparatively less than the mushrooms dried under shading plates and open sun drying.
Sra SK, Sandhu KS, Ahluwalia P. Effect of treatments and packaging on the quality of dried carrot slices during storage. J Food Sci Technol. 2014 Apr;51(4):645-54. doi: 10.1007/s13197-011-0575-x.
Abstract. The present investigation was undertaken to study the effect of treatments and packaging on the quality of dried carrot slices during storage. Carrot cultivar 'Nantes' was sliced into 4.5 mm thick slices which were blanched in water at 95 °C for 4 min followed by dipping in 6% potassium metabisulphite (KMS) solution for 40 min and 350 ppm potassium sorbate solution for 10 min prior to two stage phase drying i.e. at 90 ± 5 °C for 2 h and further drying at 60 ± 5 °C for 7 h in a cross-flow hot air cabinet dryer. The dried carrot slices were packed in 50 g packages of aluminium foil laminate (AFL) (polyethylene, aluminium foil and polyester) and high density polyethylene (HDPE) pouches having 32.5 μm and 56.0 μm thickness respectively and stored under ambient conditions i.e.18.5-29.1 °C temperature and 44.4-60.4% relative humidity for 6 months. Significant (p ≤ 0.05) increase was observed in the moisture content, water activity, reducing sugars and non-enzymatic browning while total solids, total soluble solids, titratable acidity, ascorbic acid, total sugars, pectin, rehydration ratio, sulphur dioxide, sorbic acid and carotenoids decreased significantly (p ≤ 0.05) during storage. Carrot slices pre-treated with 6% KMS and packed in AFL pouches were found to retain best physico-chemical quality. The curried product and soup prepared from dried slices from the same had highly acceptable sensory quality with initial overall acceptability scores 8.2 and 8.5 for curried slices and soup respectively on 9-point hedonic scale. The overall acceptability scores decreased from 8.2 to 7.9 and 8.5 to 7.7 in curried product and soup respectively after 6 months storage. All the samples were microbially safe during 6 months of storage.
(2) Diamante LM, Bai X, Busch J. Fruit Leathers: Method of Preparation and Effect of Different Conditions on Qualities. Int J Food Sci. 2014;2014:139890. doi: 10.1155/2014/139890.
(3) Renkema JM, Wright D, Buitenhuis R, Hallett RH. Plant essential oils and potassium metabisulfite as repellents for Drosophila suzukii (Diptera: Drosophilidae). Sci Rep. 2016 Feb 19;6:21432. doi: 10.1038/srep21432.
Abstract. Spotted wing drosophila, Drosophila suzukii, is a globally invasive pest of soft-skinned fruit. Females oviposit into ripening fruit and larvae cause direct destruction of tissues. As many plant essential oils are permitted food additives, they may provide a safe means of protecting fruit from D. suzukii infestation in both conventional and organic production systems. Twelve oils and potassium metabisulfite (KMS) were screened in the laboratory as repellents for D. suzukii flies. Most essential oils deterred D. suzukii flies from cotton wicks containing attractive raspberry juice. Peppermint oil was particularly effective, preventing almost all flies from contacting treated wicks and remaining 100% repellent for 6 d post-application. Thyme oil was unique because it caused high male mortality and reduced the number of responding flies compared to other oils. KMS was not found to be repellent to D. suzukii, but may have fumigant properties, particularly at high concentrations. Peppermint oil appears to be the best candidate for field testing to determine the effectiveness and feasibility of using essential oils as part of a push-pull management strategy against D. suzukii. This is the first time that essential oils have been evaluated and proven effective in preventing fruit-infesting flies from contacting attractive stimuli.
(4) Gunnison AF, Jacobsen DW. Sulfite hypersensitivity. A critical review. CRC Crit Rev Toxicol. 1987;17(3):185-214. doi: 10.3109/10408448709071208.
Abstract. Sulfiting agents (sulfur dioxide and the sodium and potassium salts of bisulfite, sulfite, and metabisulfite) are widely used as preservatives in foods, beverages, and pharmaceuticals. Within the past 5 years, there have been numerous reports of adverse reactions to sulfiting agents. This review presents a comprehensive compilation and discussion of reports describing reactions to ingested, inhaled, and parenterally administered sulfite. Sulfite hypersensitivity is usually, but not exclusively, found within the chronic asthmatic population. Although there is some disagreement on its prevalence, a number of studies have indicated that 5 to 10% of all chronic asthmatics are sulfite hypersensitive. This review also describes respiratory sulfur dioxide sensitivity which essentially all asthmatics experience. Possible mechanisms of sulfite hypersensitivity and sulfur dioxide sensitivity are discussed in detail. Sulfite metabolism and the role of sulfite oxidase in the detoxification of exogenous sulfite are reviewed in relationship to the etiology of sulfite hypersensitivity.
(5) EFSA Panel on Food Additives and Flavourings (FAF); Younes M, Aquilina G, Castle L, Engel KH, Fowler PJ, Frutos Fernandez MJ, Fürst P, Gundert-Remy U, Gürtler R, Husøy T, Manco M, Mennes W, Moldeus P, Passamonti S, Shah R, Waalkens-Berendsen I, Boon P, Cheyns K, Crebelli R, FitzGerald R, Lambré C, Mirat M, Ulbrich B, Vleminckx C, Mech A, Rincon AM, Tard A, Horvath Z, Wright M. Follow-up of the re-evaluation of sulfur dioxide (E 220), sodium sulfite (E 221), sodium bisulfite (E 222), sodium metabisulfite (E 223), potassium metabisulfite (E 224), calcium sulfite (E 226), calcium bisulfite (E 227) and potassium bisulfite (E 228). EFSA J. 2022 Nov 24;20(11):e07594. doi: 10.2903/j.efsa.2022.7594.
Abstract. Sulfur dioxide-sulfites (E 220-228) were re-evaluated in 2016, resulting in the setting of a temporary ADI of 0.7 mg SO2 equivalents/kg bw per day. Following a European Commission call for data, the present follow-up opinion assesses data provided by interested business operators (IBOs) and additional evidence identified in the publicly available literature. No new biological or toxicological data addressing the data gaps described in the re-evaluation were submitted by IBOs. Taking into account data identified from the literature search, the Panel concluded that there was no substantial reduction in the uncertainties previously identified in the re-evaluation. Therefore, the Panel considered that the available toxicity database was inadequate to derive an ADI and withdrew the current temporary group acceptable daily intake (ADI). A margin of exposure (MOE) approach was considered appropriate to assess the risk for these food additives. A lower confidence limit of the benchmark dose of 38 mg SO2 equivalents/kg bw per day, which is lower than the previous reference point of 70 mg SO2 equivalents/kg bw per day, was estimated based on prolonged visual evoked potential latency. An assessment factor of 80 was applied for the assessment of the MoE. At the estimated dietary exposures, when using a refined exposure scenario (Data set D), MOEs at the maximum of 95th percentile ranges were below 80 for all population groups except for adolescents. The dietary exposures estimated using the maximum permitted levels would result in MOEs below 80 in all population groups at the maximum of the ranges of the mean, and for most of the population groups at both minimum and maximum of the ranges at the 95th percentile. The Panel concluded that this raises a safety concern for both dietary exposure scenarios. The Panel also performed a risk assessment for toxic elements present in sulfur dioxide-sulfites (E 220-228), based on data submitted by IBOs, and concluded that the maximum limits in the EU specifications for arsenic, lead and mercury should be lowered and a maximum limit for cadmium should be introduced.
(6) Nair B, Elmore AR; Cosmetic Ingredients Review Expert Panel. Final report on the safety assessment of sodium sulfite, potassium sulfite, ammonium sulfite, sodium bisulfite, ammonium bisulfite, sodium metabisulfite and potassium metabisulfite. Int J Toxicol. 2003;22 Suppl 2:63-88.
Abstract. Sodium Sulfite, Ammonium Sulfite, Sodium Bisulfite, Potassium Bisulfite, Ammonium Bisulfite, Sodium Metabisulfite, and Potassium Metabisulfite are inorganic salts that function as reducing agents in cosmetic formulations. All except Sodium Metabisulfite also function as hair-waving/straightening agents. In addition, Sodium Sulfite, Potassium Sulfite, Sodium Bisulfite, and Sodium Metabisulfite function as antioxidants. Although Ammonium Sulfite is not in current use, the others are widely used in hair care products. Sulfites that enter mammals via ingestion, inhalation, or injection are metabolized by sulfite oxidase to sulfate. In oral-dose animal toxicity studies, hyperplastic changes in the gastric mucosa were the most common findings at high doses. Ammonium Sulfite aerosol had an acute LC(50) of >400 mg/m(3) in guinea pigs. A single exposure to low concentrations of a Sodium Sulfite fine aerosol produced dose-related changes in the lung capacity parameters of guinea pigs. A 3-day exposure of rats to a Sodium Sulfite fine aerosol produced mild pulmonary edema and irritation of the tracheal epithelium. Severe epithelial changes were observed in dogs exposed for 290 days to 1 mg/m(3) of a Sodium Metabisulfite fine aerosol. These fine aerosols contained fine respirable particle sizes that are not found in cosmetic aerosols or pump sprays. None of the cosmetic product types, however, in which these ingredients are used are aerosolized. Sodium Bisulfite (tested at 38%) and Sodium Metabisulfite (undiluted) were not irritants to rabbits following occlusive exposures. Sodium Metabisulfite (tested at 50%) was irritating to guinea pigs following repeated exposure. In rats, Sodium Sulfite heptahydrate at large doses (up to 3.3 g/kg) produced fetal toxicity but not teratogenicity. Sodium Bisulfite, Sodium Metabisulfite, and Potassium Metabisulfite were not teratogenic for mice, rats, hamsters, or rabbits at doses up to 160 mg/kg. Generally, Sodium Sulfite, Sodium Metabisulfite, and Potassium Metabisulfite were negative in mutagenicity studies. Sodium Bisulfite produced both positive and negative results. Clinical oral and ocular-exposure studies reported no adverse effects. Sodium Sulfite was not irritating or sensitizing in clinical tests. These ingredients, however, may produce positive reactions in dermatologic patients under patch test. In evaluating the positive genotoxicity data found with Sodium Bisulfite, the equilibrium chemistry of sulfurous acid, sulfur dioxide, bisulfite, sulfite, and metabisulfite was considered. This information, however, suggests that some bisulfite may have been present in genotoxicity tests involving the other ingredients and vice versa. On that basis, the genotoxicity data did not give a clear, consistent picture. In cosmetics, however, the bisulfite form is used at very low concentrations (0.03% to 0.7%) in most products except wave sets. In wave sets, the pH ranges from 8 to 9 where the sulfite form would predominate. Skin penetration would be low due to the highly charged nature of these particles and any sulfite that did penetrate would be converted to sulfate by the enzyme sulfate oxidase. As used in cosmetics, therefore, these ingredients would not present a genotoxicity risk. The Cosmetic Ingredient Review Expert Panel concluded that Sodium Sulfite, Potassium Sulfite, Ammonium Sulfite, Sodium Bisulfite, Ammonium Bisulfite, Sodium Metabisulfite, and Potassium Metabisulfite are safe as used in cosmetic formulations.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Chemical Main substances: Last update: 2023-08-19 21:38:09 | Chemical Risk: |