CI 75470, Carmine color is a chemical compound, a synthetic red dye and is known as Natural Red 4, Cochineal Carmine, E120.
Carmine color (also known simply as carmine or cochineal color) is a natural red pigment obtained from the dried bodies of female Dactylopius coccus, an insect native to Central and South America. The pigment is extracted by isolating carminic acid, the key chromophore responsible for its intense red hue. Carmine appears as a bright red to deep crimson powder, highly valued for its excellent stability to heat, light, and pH changes compared with most other natural colorants.

Chemical composition and structure
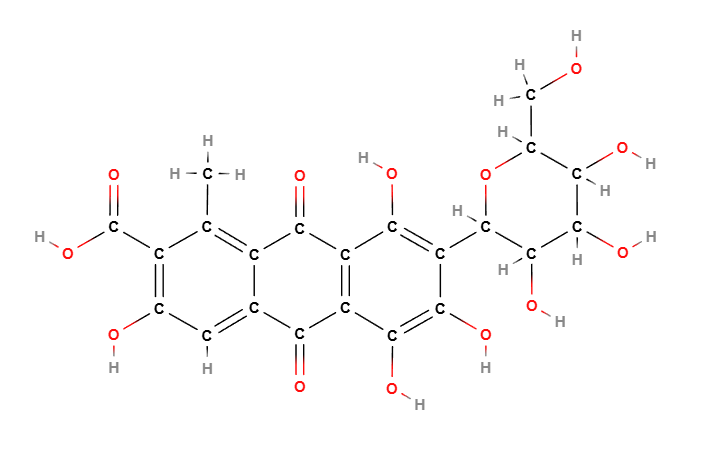
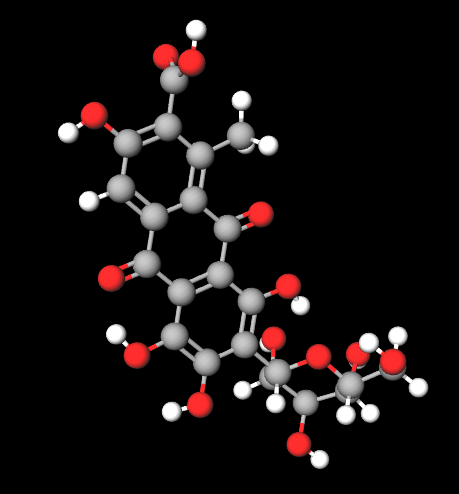
Carmine is primarily composed of carminic acid, a hydroxyanthraquinone derivative.
Major component: carminic acid (typically 40–60%).
Chemical structure: an anthraquinone backbone with sugar moieties, responsible for strong color and stability.
Other constituents: proteins, waxes, and minerals from the insect matrix, usually removed or minimized during purification.
Color form: the pigment exists mainly as a calcium or aluminum chelate of carminic acid, creating the stable “carmine lake.”
Physical and chemical properties
Appearance: red or dark red powder; solutions show bright crimson tones.
Solubility: carminic acid is soluble in water and ethanol; carmine (lake pigment) is dispersible but not fully soluble.
Stability: high resistance to heat, light, and oxidation.
pH behavior: excellent stability between pH 4–7; color shifts slightly at more acidic pH values.
Compatibility: stable in many food matrices, cosmetics, and pharmaceuticals.
Production process
The industrial production of carmine color typically involves:
Collection and drying
Extraction
Purification
Formation of the lake pigment (carmine)
Drying and milling
Applications
Environmental and safety considerations
Carmine is generally regarded as safe when used according to regulatory limits.
Toxicological profile: low toxicity; long history of use.
Allergenicity: rare but documented allergic reactions (including anaphylaxis) may occur in sensitive individuals due to insect-derived proteins; highly purified grades minimize this risk.
Environmental aspects:
Regulatory status: approved as a food colorant in many regions (e.g., E120 in the EU).
Conclusion
Carmine color is a highly stable, naturally derived red pigment with excellent coloring performance across food, cosmetic, pharmaceutical, and industrial applications. Rich in carminic acid, it offers superior stability compared with most natural dyes and remains a preferred option where strong, vivid, and durable red coloration is required. Its natural origin, long-standing safety profile, and wide functionality ensure its continued relevance in modern formulations.
Studies
It can be produced naturally by the female Coccus cacti L. (Dactylopius coccus) (1) taken from its body or by eggs, or by way of chemical synthesis and it is the most commonly observed case in food products.
In particular allergies were found (2) and the Council of Europe recommended a containment of the protein level in E120 (3) with purification systems (4).
Cochineal studies
Molecular Formula C22H20O13
Molecular Weight 492.389 g/mol
CAS: 1343-78-8
EC Number: 215-724-4
FEMA Number: 2242
UNII CID8Z8N95N
DTXSID20859613
Synonyms:
E120
Cochineal Carmine
Natural Red 4
CI 75470
References_________________________________________________________________________
(1) Perez Guerra, G. and M. Kosztarab, Biosystematics of the family Dactylopiidae (Homoptera: coccinea) with emphasis on the life cycle od Dactylopius coccus Costa, in Studies if Morphology and Systematics of Scale Insects. 1992: Blacksburg, Virginia..
Abstract. The cochineal insects include nine species assigned to the genus Dactylopius, the only genus in the family Dactylopiidae. This is a review of all the species in the family Dactylopiidae, with special emphasis on the life cycle of the type species Dactylopius coccus Costa. The adult females of the nine species have been redescribed and illustrated, with a discussion on their morphological affinities and relationships. Their hosts, natural enemies, distribution, etymology, and role as biological control agents are discussed. For several species many new distribution and host records are given. Also, new types have been designated for the following species: one neotype and three paratypes for Dactylopius coccus Costa; one neotype for D. tomentosus (Lamarck); and designation of eight new topotypes for D. opuntiae (Cockerell). Methods are given on collecting, preservation, slide mounting, measuring, and preparing illustrations. Cuticular ultrastructure is shown in scanning electron micrographs. All developmental stages of the type species, D. coccus, are described. The life cycles under two temperatures and two relative humidities, for both males and females, are discussed. Aspects of reproduction in D. coccus, its dispersal methods, factors affecting development, and its economic importance are also included. A separate chapter deals with the host-plants of Dactylopiidae. This chapter includes data on host plant suitability and host plant resistance. Three identification keys are presented: one to the suborders of Homoptera, the other to the superfamilies and families of Coccinea, and another one for the determination of the species of Dactylopius. The phylogenetic relationships of the family Dactylopiidae with respect to all the Coccinea families are discussed, and a phylogenetic tree for the Dactylopius species is proposed.
(2) Shaw DW. - Allergic contact dermatitis from carmine. Dermatitis. 2009 Sep-Oct;20(5):292-5.
Chung, K., et al., Identification of carmine allergens among three carmine allergy patients. Allergy, 2001. 56(1): p. 73-7.
Acero, S., et al., Occupational asthma and food allergy due to carmine. Allergy, 1998. 53(9): p. 897-901.
Beaudouin, E., et al., Food anaphylaxis following ingestion of carmine. Ann Allergy Asthma Immunol, 1995. 74(5): p. 427-30.
(3) en_1995L0045_do_001.pdf, h.e.e.i.e.-l.e.c.p., CONSLEG 1995L0045-10/05/2004. 2004, Official Publications of the European Communities
(4) Scopes, R.K., Protein purification : principles and practice. 3rd ed. Springer advanced texts in chemistry. 1994, New York: Springer-Verlag. xix, 380.
![]() CI 75470
CI 75470