![]() Trimethylsiloxysilicate
Trimethylsiloxysilicate
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
10 pts from Whiz35
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Trimethylsiloxysilicate studies" about Trimethylsiloxysilicate Review Consensus 9 by Whiz35 (11988 pt) | 2022-Dec-18 17:03 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Disapio A, Fridd P. Silicones: use of substantive properties on skin and hair. Int J Cosmet Sci. 1988 Apr;10(2):75-89. doi: 10.1111/j.1467-2494.1988.tb00004.x.
Abstract. Summary Silicones have been incorporated in personal care products since the 1950s. Initially used in skin care products, and more recently in hair care applications, silicones are recognized for their lubricating properties and for the characteristic soft smooth feel they impart. With recent advances in silicone technology, these fluids can also provide substantivity and durability. Resinous silicones such as trimethylsiloxysilicate act as effective substantivity additives when combined with dimethyl silicone in skin care formulations. In tests evaluating the number of wash cycles required to penetrate a silicone barrier, resistance to removal increases markedly as the proportion of resinous silicone to dimethyl silicone is increased. This improvement is related directly to decreased solubility of the resin.
Ogihara, H., Katayama, T., & Saji, T. (2011). One-step electrophoretic deposition for the preparation of superhydrophobic silica particle/trimethylsiloxysilicate composite coatings. Journal of colloid and interface science, 362(2), 560-566.
Abstract. SiO2 particle/silicone resin (trimethylsiloxysilicate (TMSS)) composite coatings were prepared by electrophoretic deposition (EPD), and their wettability was examined. SiO2 coatings prepared by EPD baths without TMSS were hydrophilic, while superhydrophobicity was observed for SiO2/TMSS composite coatings. IR spectra and EDS analyses revealed that not only SiO2 particles but also TMSS electrophoretically moved toward a cathode; as a result, hydrophilic SiO2 particles turned into superhydrophobic composite coatings by one-step EPD. SEM and AFM images of the superhydrophobic SiO2/TMSS composite coatings showed the presence of both nanometer- and micrometer-sized roughness in their surfaces. Particle size of SiO2 had a great influence on the wettability of the composite coatings. The superhydrophobic SiO2/TMSS composite coatings showed excellent water repellency; they repelled running water continuously. In addition, by controlling the amount of deposited SiO2 particles and TMSS, transparent superhydrophobic SiO2/TMSS composite coatings were prepared.
Geuli, O., & Mandler, D. (2018). The synergistic effect of benzotriazole and trimethylsiloxysilicate towards corrosion protection of printed Cu-based electronics. Corrosion Science, 143, 329-336.
Abstract. The development of printed electronics has gained much attention as an alternative for conventional metal-based electronics, mainly due to the ability to print electronic circuits on plastics and by much cheaper means as compared with conventional microelectronics. Here we report on a single stage formation of a highly corrosion resistance coating with hydrophobic properties on printed-Cu nanoparticles. Our method is based on the synergistic effect of benzotriazole (BTA) as corrosion inhibitor and trimethylsiloxysilicate (TMS) as hydrophobic component. Printed-Cu coated with such TMS/BTA layer exhibited excellent corrosion resistance in 3.5% NaCl solution, reducing the dissolution of Cu into soluble species by one order of magnitude.
Becker, L. C., Bergfeld, W. F., Belsito, D. V., Hill, R. A., Klaassen, C. D., Liebler, D., ... & Andersen, F. A. (2013). Safety assessment of silylates and surface-modified siloxysilicates. International journal of toxicology, 32(3_suppl), 5S-24S.
Abstract. The Cosmetic Ingredient Review (CIR) Expert Panel assessed the safety of silica silylate, silica dimethyl silylate, trimethylsiloxysilicate, and trifluoropropyldimethyl/trimethylsiloxysilicate as used in cosmetics. These silylates and surface-modified siloxysilicates function in cosmetics as antifoaming agents, anticaking agents, bulking agents, binders, skin-conditioning agents—emollient, skin-conditioning agents—occlusive, slip modifiers, suspension agents—nonsurfactant, and viscosity increasing agents—nonaqueous. The Expert Panel reviewed the available animal and clinical data as well as information from a previous CIR safety assessment of amorphous silica. The CIR Expert Panel concluded that silica silylate, silica dimethyl silylate, trimethylsiloxysilicate, and trifluoropropyldimethyl/trimethylsiloxysilicate are safe as used when formulated and delivered in the final product not to be irritating or sensitizing to the respiratory tract.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Trimethylsiloxysilicate Review Consensus 10 by Whiz35 (11988 pt) | 2023-Aug-15 17:08 |
| Read the full Tiiip | (Send your comment) |
Trimethylsiloxysilicate is a silicone resin, a siloxane-based compound consisting of silicon atoms (Si) bonded to methyl groups (CH3) and silicate groups (SiO3-).
The name describes the structure of the molecule:
- Trimethylsiloxysilicate is a type of silicone derivative.
- Trimethyl indicates that there are three methyl groups (CH₃) attached to the molecule.
- Siloxysilicate suggests the presence of siloxane (Si-O-Si) bonds and silicate structures in the molecule. Siloxanes are a type of silicone compound characterized by alternating silicon and oxygen atoms.
Description of the raw materials used in its production:
- Trimethylchlorosilane (TMCS) - Trimethylchlorosilane is a key raw material used in the synthesis of trimethylsiloxysilicate. It serves as a source of the trimethylsilyl (-Si(CH3)3) functional group in the compound.
- Silicate precursors - Silicate precursors, such as tetraethyl orthosilicate (TEOS) or tetramethyl orthosilicate (TMOS), may be used as reactants to provide the silicate groups (SiO3-) in the trimethylsiloxysilicate molecule.
Summary of the industrial synthesis process step-by-step:
- Preparation of trimethylsiloxane - Trimethylsiloxane is prepared by reacting trimethylchlorosilane with water (H2O) or an alcohol in the presence of a catalyst, such as an acid or a base. This reaction leads to the formation of a silanol (-Si-OH) group.
- Condensation reaction - The silanol groups in the trimethylsiloxane undergo a condensation reaction, either by self-condensation or in the presence of silicate precursors. This reaction results in the formation of siloxane bonds (Si-O-Si) and the release of water molecules.
- Addition of crosslinkers - Crosslinkers, such as tetraethoxysilane (TEOS) or tetramethoxysilane (TMOS), may be added to the reaction mixture to introduce additional silicate groups and promote crosslinking within the trimethylsiloxysilicate molecule.
- Polymerization - The reaction mixture is allowed to undergo further polymerization, resulting in the formation of a polymeric network of trimethylsiloxysilicate.
- Purification - The synthesized trimethylsiloxysilicate is purified to remove any impurities or by-products through processes such as filtration or distillation.
- Characterization - The purified trimethylsiloxysilicate is characterized using various analytical techniques to confirm its identity, purity, and desired properties.
- Incorporation into products - The trimethylsiloxysilicate is incorporated into various formulations, such as cosmetics or coatings, where its adhesive and film-forming properties are utilized.
- Packaging and distribution - The final product, containing trimethylsiloxysilicate, is packaged and distributed for use in various industries.
It appears in the form of a white powder insoluble in water, soluble in alcohol, toluene, xylene, hexamethyldisiloxane, cyclosiloxane, and low-viscosity silicone oil.

What it is used for and where
Cosmetics
Antifoaming agent. The constituent factors for foam stabilisation are the concentration of nanoparticles and hydrophobicity. Foam, even when used in separation operations such as fractionation or flotation, can cause a decrease in density and a deterioration in quality in cosmetic products. The defoaming agent (non-polar oil, silicone oils, hydrophobic solid particles or mixtures of both) is strongly influenced by viscosity and, to an almost directly proportional extent, concentration. However, defoamers can carry an irreversible source of contamination.
Skin conditioning agent - Emollient. Emollients have the characteristic of enhancing the skin barrier through a source of exogenous lipids that adhere to the skin, improving barrier properties by filling gaps in intercorneocyte clusters to improve hydration while protecting against inflammation. In practice, they have the ability to create a barrier that prevents transepidermal water loss. Emollients are described as degreasing or refreshing additives that improve the lipid content of the upper layers of the skin by preventing degreasing and drying of the skin. The problem with emollients is that many have a strong lipophilic character and are identified as occlusive ingredients; they are oily and fatty materials that remain on the skin surface and reduce transepidermal water loss. In cosmetics, emollients and moisturisers are often considered synonymous with humectants and occlusives.
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
Provides a flexible surface film for many skin care products. Excellent water repellent resistant to washing detergent used in facial products, skin, sunscreen, creams, lotions.
Dosage:
- Skin care (face, body): 2% - 4%
- Colour cosmetics (lipsticks, lip glosses, eyeliners, BB creams): 11% -14%.
- Hair care: 2% - 10%.
- Deodorants and antiperspirants: 3% - 10%.
- Sun protection: 1% -5%.
Use: premix with a solvent, such as cyclopentasiloxane or low-viscosity silicone oil, then add the premix to the oily phase.
Safety
The Cosmetic Ingredient Review (CIR) Expert Panel evaluated the safety of Trimethylsiloxysilicate and found it to be safe in current use practices and at the concentrations described in the safety assessment when formulated and delivered in the final product to be non-irritating or sensitizing to the respiratory tract (1).
The most relevant studies on this ingredient have been selected with a summary of their contents:
Trimethylsiloxysilicate studies
Typical commercial product characteristics Trimethylsioxysilicate
| Appearance | White powder |
| Boiling Point | 203.1ºC at 760mmHg |
| Flash Point | 76.7ºC |
| Density | 1.134g/cm3 |
| PSA | 46.53000 |
| LogP | 0.24560 |
| Volatility | ≤2% |
| Purity | HPLC>99.5% |
| Resin | 100% |
| Particle Size | <80 microns |
| Shelf life | 24 months |
 |  |
 |  |
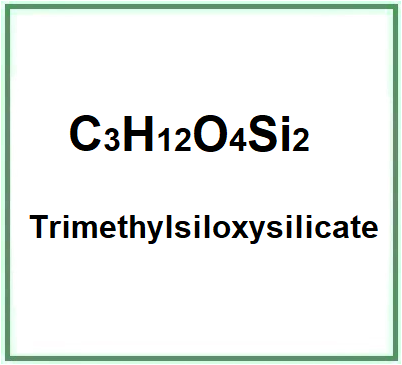
- Molecular Formula C3H12O4Si2
- Molecular Weight 168.30
- Exact Mass 150.28100
- CAS 56275-01-5
- UNII OU6EUR7UAY
- EC Number 213-997-4
- DSSTox Substance ID DTXSID9044958
- IUPAC trihydroxy(trimethylsilyloxy)silane
- InChl=1S/C3H12O4Si2/c1-8(2,3)7-9(4,5)6/h4-6H,1-3H3
- InChl Key MNTALGTVSQTALQ-UHFFFAOYSA-N
- SMILES C[Si](C)(C)O[Si](O)(O)O
- MDL number
- PubChem Substance ID
- ChEBI
- RXCUI 1426677
Synonyms:
- trimethylsilyl silicate
- trihydroxy(trimethylsilyloxy)silane
- Silicicacid,trimethylsilylester
- Silicic acid, trimethylsilyl ester
- Hydrolysisproductoftrimethylchlorosilane-ethylsilicateandwater
- MQresin
- MQ Resin
References_____________________________________________________________________
(1) Becker, L. C., Bergfeld, W. F., Belsito, D. V., Hill, R. A., Klaassen, C. D., Liebler, D., ... & Andersen, F. A. (2013). Safety assessment of silylates and surface-modified siloxysilicates. International journal of toxicology, 32(3_suppl), 5S-24S.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2022-12-18 16:13:10 | Chemical Risk: |