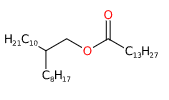
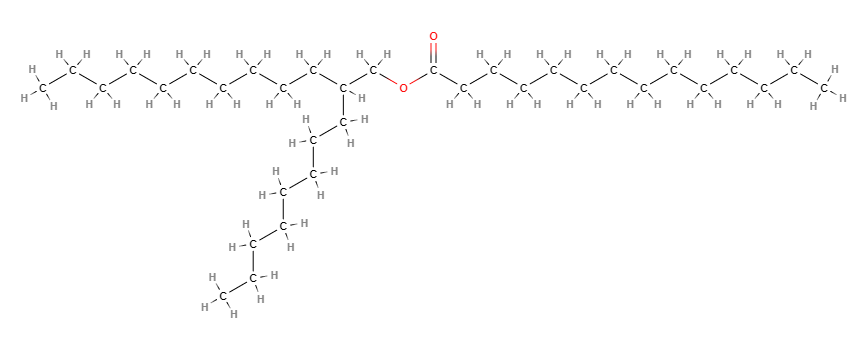
Octyldodecyl myristate is a chemical compound derived from the reaction of myristic fatty acid with octyldodecanol, an ester of octyldodecyl alcohol and myristic acid.
The name describes the structure of the molecule:
- Octyldodecyl. This term refers to an alkyl group, which is a chain of carbon and hydrogen atoms. In the case of Octyldodecyl, the chain contains 20 carbon atoms.
- Myristate. This refers to the salt or ester of myristic acid, a saturated fatty acid commonly found in nutmeg, butter, and other animal fats.
The synthesis process takes place in different steps:
- Preparation of Starting Materials. The synthesis of Octyldodecyl Myristate begins with the preparation of the necessary starting materials. This includes octyldodecyl alcohol and myristic acid.
- Esterification. The octyldodecyl alcohol is then reacted with myristic acid in the presence of an acid catalyst. This process is known as esterification and produces Octyldodecyl Myristate.
- Purification. The final step in the synthesis process is purification. This involves removing any unreacted starting materials and byproducts from the reaction mixture to obtain the pure Octyldodecyl Myristate.
It appears as a colourless transparent liquid that is insoluble in water.

What it is used for and where
Cosmetics
Skin conditioning agent - Emollient. Emollients have the characteristic of enhancing the skin barrier through a source of exogenous lipids that adhere to the skin, improving barrier properties by filling gaps in intercorneocyte clusters to improve hydration while protecting against inflammation. In practice, they have the ability to create a barrier that prevents transepidermal water loss. Emollients are described as degreasing or refreshing additives that improve the lipid content of the upper layers of the skin by preventing degreasing and drying of the skin. The problem with emollients is that many have a strong lipophilic character and are identified as occlusive ingredients; they are oily and fatty materials that remain on the skin surface and reduce transepidermal water loss. In cosmetics, emollients and moisturisers are often considered synonymous with humectants and occlusives.
Skin conditioning agent - Occlusive. This ingredient has the task of modifying the condition of the skin when it is damaged or dry by reducing flaking and restoring elasticity. It has a strong lipophilic character and is identified as an occlusive ingredient; it is generally composed of oily and fatty materials that remain on the skin surface and reduce trans epidermal water loss.
An emollient, softening, moisturising agent used in skin care products, toiletries and herbal products such as night creams and waxes.
Use: 2%
Insecticides
Formulation consisting of 5% oil (octyldodecyl myristate), 5% surfactants (sorbitan monooleate/polysorbate 80), 5% apolar fraction from M. subsericea and 85% water (1).
The most relevant studies on this chemical compound have been selected with a summary of their contents:
Octyldodecyl myristate studies
Typical commercial product characteristics Octyldodecyl myristate
| Appearance | Colourless liquid |
| Boiling Point | 522.4ºC at 760mmHg |
| Melting Point | 42°C-46°C |
| Flash Point | 275.9ºC |
| Density | 0.857g/cm3 |
Saponification value
| 103 ÷ 118 |
| K.F. water | 1% max |
Acidity value
| 1 max |
Hydroxyl value
| 2 max |
| Specific gravity | 0.85 (H2O=1) |
| Vapor pressure | >1 |
| Storage | <30°C |
- Molecular Formula C34H68O2
- Molecular Weight 508.9
- Exact Mass 508.52200
- CAS 22766-83-2, 83826-43-1
- UNII S013N99GR8
- EC Number 245-205-8
- DSSTox Substance ID
- IUPAC 2-octyldodecyl tetradecanoate
- InChI=1S/C34H68O2/c1-4-7-10-13-16-18-19-20-22-25-28-31-34(35)36-32-33(29-26-23-15-12-9-6-3)30-27-24-21-17-14-11-8-5-2/h33H,4-32H2,1-3H3
- InChl Key BGRXBNZMPMGLQI-UHFFFAOYSA-N
- SMILES CCCCCCCCCCCCCC(=O)OCC(CCCCCCCC)CCCCCCCCCC
- MDL number
- PubChem Substance ID
- SCHEMBL33795
- RXCUI 1311617
- RTECS
Synonyms:
- 2-octyldodecyl tetradecanoate
- Myristic acid, 2-octyldodecyl ester
- 2-Octyldodecyl myristate
References______________________________________________________________________
(1) Fernandes CP, de Almeida FB, Silveira AN, Gonzalez MS, Mello CB, Feder D, Apolinário R, Santos MG, Carvalho JC, Tietbohl LA, Rocha L, Falcão DQ. Development of an insecticidal nanoemulsion with Manilkara subsericea (Sapotaceae) extract. J Nanobiotechnology. 2014 May 18;12:22. doi: 10.1186/1477-3155-12-22.
Kim, E. J., Kong, B. J., Kwon, S. S., Jang, H. N., & Park, S. N. (2014). Preparation and characterization of W/O microemulsion for removal of oily make‐up cosmetics. International Journal of cosmetic science, 36(6), 606-612.
![]() Octyldodecyl myristate
Octyldodecyl myristate