Decyl Oleate is a chemical compound produced by the reaction of decanol and oleic acid.
The name describes the structure of the molecule:
- Decyl is a decyl functional group with chemical formula -C10H21. It is derived from decane by removal of a hydrogen atom.
- Oleate is an oleate ion, derived from oleic acid, a type of monounsaturated fatty acid. Oleic acid has the chemical formula C18H34O2 and, when it loses a hydrogen ion (H+), it becomes an oleate ion (C18H33O-2).
The synthesis process takes place in different steps:
- Production of decyl alcohol and oleic acid. The first step concerns the production of the two components that will make up the ester: decyl alcohol and oleic acid. Decyl alcohol can be produced by the hydrogenation of decyl aldehyde, which is derived from the hydroformylation of 1-nonene. Oleic acid can be obtained from natural sources such as vegetable oils, or it can be synthesised from stearic acid.
- Esterification. After production, decyl alcohol and oleic acid can be fed into a reaction process to form the ester. This is done by heating the two components in the presence of a catalyst such as sulphuric acid to accelerate the reaction. The reaction produces decyl oleate and water.
- Purification. The decyl oleate thus obtained is purified to remove unreacted decyl alcohol or oleic acid, the catalyst and any other impurities. This is generally done by a process such as distillation or filtration.
It appears as a yellow liquid.

What it is used for and where
Cosmetics
It is used by the cosmetic industry as an emollient in moisturizers, creams, sunscreens, lip balms, anti-aging serums and various makeup products and has become a typical component in beauty and body care products as it makes the skin smooth and pleasant to the touch.
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
Skin conditioning agent - Emollient. Emollients have the characteristic of enhancing the skin barrier through a source of exogenous lipids that adhere to the skin, improving barrier properties by filling gaps in intercorneocyte clusters to improve hydration while protecting against inflammation. In practice, they have the ability to create a barrier that prevents transepidermal water loss. Emollients are described as degreasing or refreshing additives that improve the lipid content of the upper layers of the skin by preventing degreasing and drying of the skin. The problem with emollients is that many have a strong lipophilic character and are identified as occlusive ingredients; they are oily and fatty materials that remain on the skin surface and reduce transepidermal water loss. In cosmetics, emollients and moisturisers are often considered synonymous with humectants and occlusives.
Regarding skin permeation, this study found that microemulsions prepared using the most lipophilic lipids (decyl oleate or cetyl stearyl isonononanoate) showed the lowest stability (1).
Medical
Solid lipid nanoparticle (SLN) has been considered as a novel topical delivery system for pharmaceutical and cosmetic active ingredients, and this study developed delivery systems for organic and inorganic sunscreens based on a matrix composed of carnauba wax and decyl oleate (2).
Other uses
It is an excellent lubricant as it has a low viscosity.
The most relevant studies on this chemical compound have been selected with a summary of their contents:
Decyl Oleate studies
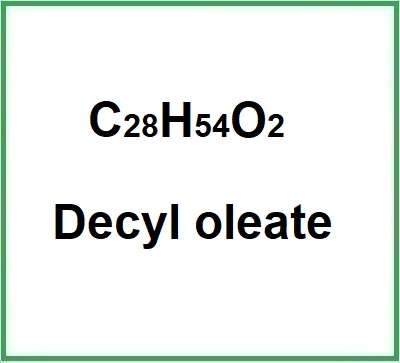
- Molecular Formula: C28H54O2
- Molecular Weight: 422.7
- CAS: 3687-46-5
- UNII ZGR06DO97T
- EC Number: 222-981-6
- DSSTox Substance ID: DTXSID1029259
Synonyms:
- Decyloleate
- SCHEMBL15078
- 9-Octadecenoic acid(9Z)-, decyl ester
- Oleic acid, decyl ester
- Tegosoft DO
- Ceraphyl 140
References________________________________________________________________________
(1) Montenegro L, Carbone C, Puglisi G. Vehicle effects on in vitro release and skin permeation of octylmethoxycinnamate from microemulsions. Int J Pharm. 2011 Feb 28;405(1-2):162-8. doi: 10.1016/j.ijpharm.2010.11.036.
Abstract. The high content of surfactants is one of the major limits to microemulsions (MEs) use in pharmaceutical and cosmetic field. In this work MEs with low surfactant content were prepared by the phase inversion temperature (PIT) method using different oil phases and emulsifiers. The effects of vehicle composition on in vitro release and skin permeation of octylmethoxycinnamte (OMC), one of the most used UVB filter, was evaluated. These MEs showed droplet sizes in the range 32-77nm and a single peak in size distribution. MEs prepared using the most lipophilic lipids (decyl oleate or cetyl stearyl isononanoate) showed the lowest stability. In vitro release and skin permeation profiles were affected by both lipophilicty and structure of the lipid used as internal phase and the formulation that released the lowest amount of OMC provided the lowest active compound skin permeation. It is noteworthy that no OMC release and skin permeation were observed using oleth-20/glyceryl oleate as emulsifiers. Furthermore, a skin permeation enhancement effect was observed depending on the vehicle components. The results of this work suggest that PIT MEs could provide controlled skin drug delivery by choosing proper associations of oil phase lipids and emulsifiers. Copyright © 2010 Elsevier B.V. All rights reserved.
(2) Nesseem D. Formulation of sunscreens with enhancement sun protection factor response based on solid lipid nanoparticles. Int J Cosmet Sci. 2011 Feb;33(1):70-9. doi: 10.1111/j.1468-2494.2010.00598.x.
Abstract. Solid lipid nanoparticle (SLN) was regarded as new topical delivery systems for pharmaceutical and cosmetic active ingredients. The purpose of this study is to develop carrier systems for organic and inorganic sunscreens based on a matrix composed of carnauba wax and decyl oleate. Formulae (F1-F7) were prepared using butyl methoxydibenzoylmethane and octyl methoxycinnamate as organic components, and titanium dioxide (TiO(2) ) was used as inorganic component. Both types of sunscreens were incorporated into SLN formulations using classical method of preparation. To evaluate the effect of the pigments on the nanoparticles, particle size was measured using Mastersizer particle size analyser. UV-protection abilities of formulations were investigated by the in vitro sun protection factor test (SPF). Further parameters determined were spreadability as well as viscosity. The rheological behaviour of the formulations was also carried out. From the plot of log of shear stress vs. log of shear rate, the slope of the plot representing flow index and ontology of the y-intercept indicating consistency index was calculated. The formulae showed a flow index of 0.2074-0.4005 indicating pseudoplastic flow behaviour. Significant increases in SPF values up to about 50 were reported after the encapsulation by using organic and inorganic filters in Canada wax and decyl oleate. So, SLN could be appropriate vehicles to carry organic and inorganic sunscreens. The rational combination of cinnamates, titanium dioxide and Zinc oxide has shown a synergistic effect to improve the SPF of cosmetic preparations. © 2010 The Author. Journal compilation. © 2010 Society of Cosmetic Scientists and the Société Française de Cosmétologie.
![]() Decyl oleate
Decyl oleate