![]() Quinine hydrochloride
Quinine hydrochloride
Rating : 5.7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
(1)Cons:
Possible risk. Click on ingredient (1)10 pts from A_Partyns
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Quinine studies" about Quinine hydrochloride Review Consensus 8 by A_Partyns (13112 pt) | 2021-Jan-01 16:54 |
| Read the full Tiiip | (Send your comment) |
The best studies on quinine with regard to applications, intake and effects.
Analytics of Quinine and its Derivatives.
Kluska M, Marciniuk-Kluska A, Prukała D, Prukała W
Crit Rev Anal Chem. 2016;46(2):139-45. doi: 10.1080/10408347.2014.996700.

Artesunate versus Quinine - keeping our options open.
Frosch AEP.
Clin Infect Dis. 2019 May 2. pii: ciz335. doi: 10.1093/cid/ciz335.
Protective effects of pyrroloquinoline quinine against oxidative stress-induced cellular senescence and inflammation in human renal tubular epithelial cells via Keap1/Nrf2 signaling pathway.
Wang Z, Han N, Zhao K, Li Y, Chi Y, Wang B.
Int Immunopharmacol. 2019 Jul;72:445-453. doi: 10.1016/j.intimp.2019.04.040.
Synthesis and systemic toxicity assessment of quinine-triazole scaffold with antiprotozoal potency.
Sahu A, Agrawal RK, Pandey R.
Bioorg Chem. 2019 Apr 20;88:102939. doi: 10.1016/j.bioorg.2019.102939.
Shape adaptation of quinine in cyclodextrin cavities: NMR studies.
Wójcik J, Ejchart A, Nowakowski M.
Phys Chem Chem Phys. 2019 Mar 27;21(13):6925-6934. doi: 10.1039/c9cp00590k.
Pyrroloquinoline quinine protects HK-2 cells against high glucose-induced oxidative stress and apoptosis through Sirt3 and PI3K/Akt/FoxO3a signaling pathway.
Wang Z, Li Y, Wang Y, Zhao K, Chi Y, Wang B.
Biochem Biophys Res Commun. 2019 Jan 8;508(2):398-404. doi: 10.1016/j.bbrc.2018.11.140.
Effects of Quinine, Quinidine and Chloroquine on Human Muscle Nicotinic Acetylcholine Receptors.
Gisselmann G, Alisch D, Welbers-Joop B, Hatt H.
Front Pharmacol. 2018 Nov 20;9:1339. doi: 10.3389/fphar.2018.01339. eCollection 2018.
High-level artemisinin-resistance with quinine co-resistance emerges in P. falciparum malaria under in vivo artesunate pressure.
Tyagi RK, Gleeson PJ, Arnold L, Tahar R, Prieur E, Decosterd L, Pérignon JL, Olliaro P, Druilhe P.
BMC Med. 2018 Oct 1;16(1):181. doi: 10.1186/s12916-018-1156-x.

Use of hyperbaric oxygen therapy in quinine-associated visual disturbances.
Laes JR, Hendriksen S, Cole JB.
Undersea Hyperb Med. 2018 Jul-Aug;45(4):457-461.
fMRI-Based Brain Responses to Quinine and Sucrose Gustatory Stimulation for Nutrition Research in the Minipig Model: A Proof-of-Concept Study.
Coquery N, Meurice P, Janvier R, Bobillier E, Quellec S, Fu M, Roura E, Saint-Jalmes H, Val-Laillet D.
Front Behav Neurosci. 2018 Jul 24;12:151. doi: 10.3389/fnbeh.2018.00151. eCollection 2018.
A Case Report of Nephrotic Syndrome While Undergoing Quinine Therapy.
Albrecht B, Giebel S, McCarron M, Prasad B.
Cureus. 2018 Mar 7;10(3):e2283. doi: 10.7759/cureus.2283.

The Role of Quinine-Responsive Taste Receptor Family 2 in Airway Immune Defense and Chronic Rhinosinusitis.
Workman AD, Maina IW, Brooks SG, Kohanski MA, Cowart BJ, Mansfield C, Kennedy DW, Palmer JN, Adappa ND, Reed DR, Lee RJ, Cohen NA.
Front Immunol. 2018 Mar 28;9:624. doi: 10.3389/fimmu.2018.00624. eCollection 2018.
Intragastric quinine administration decreases hedonic eating in healthy women through peptide-mediated gut-brain signaling mechanisms.
Iven J, Biesiekierski JR, Zhao D, Deloose E, O'Daly OG, Depoortere I, Tack J, Van Oudenhove L.
Nutr Neurosci. 2018 Apr 2:1-13. doi: 10.1080/1028415X.2018.1457841.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Quinine in bitter drinks" about Quinine hydrochloride Review Consensus 8 by A_Partyns (13112 pt) | 2022-May-20 17:39 |
| Read the full Tiiip | (Send your comment) |
The best study I have yet met on the indications and contraindications of quinine in bitter drinks, is undoubtedly the one published in 2008 by BfR which is the German Federal Institute for Risk Assessment and was established as a federal authority under the jurisdiction of the Federal Ministry of Food and German agriculture (BmEL). Here is the first part. The study is very long, about 16 pages and contains all the bibliographical references:
Quinine is a bitter-tasting, crystalline white powder. It is obtained from the bark of the cinchona tree and belongs to the group of alkaloids. In medicine quinine is used to treat malaria and nocturnal leg cramps. In the food sector, quinine is used as a flavouring mainly in beverages like bitter lemon and tonic water.

When larger amounts of quinine are consumed, it can constitute a health problem for some consumer groups. BfR sees risks in particular for quinine intakes during pregnancy. For instance, a newborn baby, whose mother had drunk more than 1 litre tonic water a day in the weeks up to its birth, suffered health disorders. Based on existing regulations in the medicinal product sector, BfR, therefore, advises pregnant women against drinking quininecontaining beverages on precautionary grounds. People who have been advised against taking quinine, cinchona bark or their preparations by their doctors because of their clinical pictures should not consume any quinine-containing soft drinks either. This applies, for instance, to people who suffer from tinnitus, pre-existing damage to the optic nerve, haemolytic anaemia or who are hypersensitive to quinine or cinchona alkaloids. Patients with cardiac arrhythmia and people who take medicine that interacts with quinine, should only drink quinine-containing soft drinks after consulting their doctors. This applies in particular to medications which inhibit blood coagulation. At higher levels of tonic water consumption, it may be necessary to reduce their therapeutic dose.

Already today quinine must be mentioned by name in the list of ingredients of quininecontaining products. BfR also believes that there is a need for information which attracts the attention more particularly of pregnant women and other risk groups to possible health impairments. Motor vehicle drivers should be informed that larger amounts of quininecontaining bitter beverages can cause visual disturbances. BfR recommends raising awareness about the possible health risks from quinine to consumers. Specific information should be provided about the symptoms of quinine hypersensitivity and cinchonism (typical adverse reactions to quinine). Consumers should be advised to immediately stop their quinine intake if these symptoms occur, and to consult a doctor.
BfR recommends that the health assessment of quinine by the Scientific Committee on Food from 1988 should be updated.
BfR is of the opinion that the problems of quinine-containing bitter soft drinks underline the importance of the systematic recording of adverse reactions that occur in conjunction with the consumption of foods. The Institute, therefore, explicitly supports the setting up of a central reporting office (1).
References___________________________________________________
(1) Quinine-containing beverages may cause health problems
Updated BfR Health Assessment No 020/2008, 17 February 2005
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Quinine hydrochloride Review Consensus 10 by A_Partyns (13112 pt) | 2026-Jan-11 16:11 |
| Read the full Tiiip | (Send your comment) |
Quinine hydrochloride: : properties, uses, pros, cons, safety
Quinine hydrochloride (monohydrochloride; commonly supplied as dihydrate) – hydrochloride salt of quinine, an alkaloid historically obtained from Cinchona spp. (Rubiaceae)
Synonyms: quinine HCl, quinine monohydrochloride, quinine hydrochloride dihydrate, chinona (legacy/trade usage), quinine salt (generic)
INCI / Functions (CosIng reference for “Quinine”): denaturant, hair conditioning, fragrance

Definition
Quinine hydrochloride is the hydrochloride salt of quinine, a naturally occurring alkaloid best known for its historical antimalarial use and for its intensely bitter sensory profile. In practice, the salt form is used to improve handling and standardization versus the free base, and it is frequently marketed and supplied as a hydrate (most commonly the dihydrate), which matters for analytical control and formulation repeatability.
From a use-case standpoint, quinine hydrochloride sits at the intersection of (1) pharmaceutical quality systems (where assay, impurities, and hydrate state are tightly managed), (2) technical/cosmetic positioning (often linked to denaturation/bittering and legacy “hair tonic” concepts), and (3) analytical laboratory use, including applications where quinine’s spectroscopic behavior (notably fluorescence) is operationally useful. In modern personal care, its role is typically supporting/technical rather than being a primary performance driver, and any consumer-facing narrative must remain consistent with cosmetic claim boundaries and the safety assessment of the finished product.
Main uses
Food and beverages.
Quinine and its salts are used primarily for bittering and flavor profiling in specific beverage categories (classically “bitter drinks”). From a regulatory and consumer-information standpoint, quinine is a substance for which presence disclosure on labeling has been specifically addressed in EU rules for certain scenarios. In practice, this means the compliance focus is less about “functionality” (the bitterness is straightforward) and more about correct labeling language, category fit, and adherence to applicable maximum levels and national implementations where relevant.
Cosmetics and personal care.
Within cosmetics, quinine appears in regulatory databases under INCI “Quinine” with functions that include denaturant, hair conditioning, and fragrance. The most technically defensible function is typically denaturant/bittering for products where accidental ingestion is a concern (e.g., certain alcohol-containing solutions), because quinine’s bitterness is robust at very low levels. In hair products, quinine may be used in legacy “tonic” concepts; however, its practical contribution is highly formulation-dependent and should not be positioned with drug-like efficacy claims. In any case, compatibility (pH, salts, polymers) and stability (light/oxygen) should be verified in the specific matrix.
Medical use.
Quinine is historically associated with antimalarial therapy and remains a substance with medically relevant pharmacology. The hydrochloride salt is one of the well-known salified forms used in medicinal contexts. For a cosmetic or technical dossier, the key point is that “medical history” does not translate into cosmetic claims; it mainly informs a conservative approach to safety evaluation, contraindications awareness, and communication discipline.
Pharmaceutical use.
In pharmaceutical settings, quinine hydrochloride can be an API in defined contexts or a controlled substance under pharmacopeial expectations (depending on jurisdiction and product type). The dominant themes are quality control (assay, impurity profile, residual solvents where applicable) and consistent control of hydrate state (because dihydrate vs anhydrous directly changes molecular weight basis and assay calculations). Supply qualification and CoA alignment to compendial requirements are typically non-negotiable.
Industrial and laboratory use.
Quinine hydrochloride is widely used as a laboratory chemical and reference material, including analytical workflows where quinine’s fluorescence properties are leveraged (for instrument checks, method development, or teaching labs). Industrial use is therefore often “R&D/QC driven” rather than high-volume. Here, handling safety (powder exposure control) and traceability (lot, assay basis, hydrate form) are the core operational priorities.
Identification data and specifications
| Identifier | Value |
|---|---|
| INCI name (practical reference) | Quinine |
| Substance name | Quinine hydrochloride |
| Common commercial form | Dihydrate (common); anhydrous (less common) |
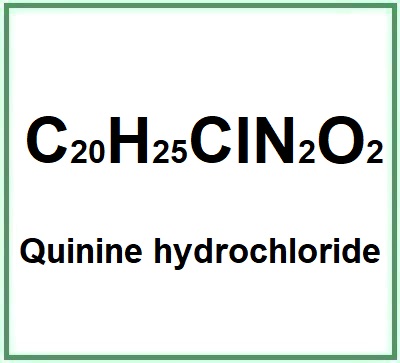
| Formula (anhydrous) | C20H25ClN2O2 |
| Molecular weight (anhydrous) | 360.88 g/mol |
| Formula (dihydrate) | C20H25ClN2O2 · 2H2O |
| Molecular weight (dihydrate) | ~396.91–396.92 g/mol (grade-dependent) |
| CAS number (anhydrous) | 130-89-2 |
| EC/EINECS number (anhydrous) | 205-001-1 |
| CAS number (dihydrate) | 6119-47-7 |
| EC/EINECS number (dihydrate) | 612-097-2 |
| Typical commercial appearance | White to off-white powder/crystals (grade-dependent) |
Chemical-physical properties (indicative)
| Property | Value | Note |
|---|---|---|
| Water solubility | High (grade-dependent) | Salt form and hydration state influence supplier data |
| pH (10 g/L, H2O, 20 °C) | ~6.0 | Commonly reported for certain dihydrate grades |
| Sensitivity | Potential discoloration with light/oxygen | Confirm stability and consider protective packaging if needed |
Functional role and practical mechanism
| Function | What it does in formula | Technical note |
|---|---|---|
| Denaturant / bitterant | Provides strong bitterness to discourage ingestion | Typical at very low levels in suitable matrices |
| Hair conditioning | Function listed in regulatory databases | Outcome depends on the full formula and use level |
| Fragrance | Secondary sensory contribution | Bitterness is often more relevant than odor |
Formulation compatibility
Quinine hydrochloride is an ionic organic salt, so compatibility is mainly governed by pH, ionic strength, and interactions with charged polymers/surfactant systems. If pH shifts upward, equilibrium can move toward the free base, increasing the risk of haze or precipitation, particularly in electrolyte-rich or polymer-rich matrices. Define a controlled pH window and verify clarity under temperature cycling.
In transparent hydroalcoholic systems, light stability is often a practical constraint: discoloration can become a quality issue even when the ingredient remains within chemical specifications. Packaging selection and accelerated stability testing are recommended when appearance is critical.
Use guidelines (indicative)
| Application | Typical range | Technical note |
|---|---|---|
| Alcoholic/technical solutions (denaturation/bittering) | ppm – 0.01% | Target is controlled bitterness, not cosmetic “active” effect |
| Hair products (rinse-off / leave-on) | Variable, often low | Define based on stability, claim strategy, and finished-product safety |
| Laboratory/QC use | Per method/monograph | Manage traceability, assay basis, and hydration state |
Quality, grades, and specifications
| QC parameter | What to check |
|---|---|
| Identity | Correct naming, form (anhydrous/dihydrate), CAS/EC alignment |
| Assay | Content and calculation basis (hydrate state matters) |
| Water/hydration | Loss on drying; confirmation of dihydrate state where applicable |
| Impurities | Profile consistent with intended use (cosmetic/pharma/lab) |
| Appearance | Color and changes over time (light stability) |
Safety, regulatory, and environmental notes
Supplier SDS commonly classifies quinine hydrochloride as harmful if swallowed and potentially sensitizing (skin and/or respiratory). Powder handling should therefore be controlled with appropriate containment and PPE. Cosmetic safety substantiation must be performed on the finished product (exposure, product type, target population), consistent with GMP (first occurrence) expectations. Potential benefit: improved batch consistency and traceability.
In the EU food/beverage context, quinine is associated with specific consumer information rules in certain scenarios, including clear labeling expectations. HACCP (first occurrence) concepts may be relevant in regulated supply chains. This study warns of the dangers associated with a high intake of quinine in beverages (1)
Formulation troubleshooting
| Problem | Possible cause | Recommended intervention |
|---|---|---|
| Haze/precipitation | High pH; salt/polymer interactions; incomplete dissolution | Adjust pH, reduce electrolytes, optimize addition order and dissolution |
| Discoloration | Light/oxygen sensitivity; unsuitable packaging | Run light testing, use protective packaging, reduce process exposure |
| Lot-to-lot variability | Differences in hydration/purity across grades | Set LOD/hydration specs, qualify suppliers, tighten CoA requirements |
| Excess bitterness | Overdosing or poor distribution | Lower level, improve dissolution, reassess sensory target |
Conclusion
Quinine hydrochloride is most defensible in modern formulations as a denaturant/bitterant, with additional legacy positioning in certain hair concepts. Key technical control points are hydrate state, pH-dependent solubility, and appearance stability (notably light exposure). Compliance depends on robust finished-product safety assessment for cosmetics and correct labeling alignment where quinine is used in foods/beverages.
Mini-glossary
INCI: Standardized cosmetic ingredient naming system.
Denaturant: Substance added to discourage ingestion and deter misuse.
Assay: Quantitative determination of main component content.
CoA: Certificate of Analysis; batch-specific supplier certificate.
SDS: Safety Data Sheet; hazards and handling document.
GMP: Good Manufacturing Practice; quality system for consistent manufacturing and control.
HACCP: Hazard Analysis and Critical Control Points; risk-based control system used in regulated environments.
- Molecular Formula: C20H25ClN2O2 C20H24N2O2 · HCl · 2H2O
- Molecular Weight: 360.882 g/mol
- UNII: 7CS0WNO31M
- CAS: 130-89-2 6119-47-7 7549-43-1 11034-23-4 70181-37-2 70181-38-3 918656-55-0
- EC Number: 231-437-7 205-001-1
- FEMA Number: 2976
- PubChem Substance ID 329751012
- MDL number MFCD00151248
- Beilstein Registry Number 6112655
Synonyms:
- (8alpha,9R)-6'-Methoxycinchonan-9-Ol Monohydrochloride
- Quinine HCl
- Quinine hydrochloride
- Cinchonan-9-ol, 6'-methoxy-, hydrochloride, (8alpha,9R)-
References__________________________________________________________________________
(1) https://www.bfr.bund.de/cm/349/quinine_containing_beverages_may_cause_health_problems.pdf
Colley JC, Edwards JA, Heywood R, Purser D. Toxicity studies with quinine hydrochloride. Toxicology. 1989 Feb;54(2):219-26. doi: 10.1016/0300-483x(89)90047-4.
Abstract. Three-month studies in the rat, a rat embryo-toxicity study and a specific study to investigate ototoxicity were carried out with quinine hydrochloride. The results of these studies suggest an acceptable daily intake of 40 mg quinine hydrochloride for an adult. There were no indications of teratogenic effects and no indications of interference with auditory function in rats receiving up to 200 mg/kg.
Trenholme GM, Williams RL, Rieckmann KH, Frischer H, Carson PE. Quinine disposition during malaria and during induced fever. Clin Pharmacol Ther. 1976 Apr;19(4):459-67. doi: 10.1002/cpt1976194459.
Abstract. Quinine disposition was studied in 5 subjects before and during an experimentally induced infection with a chloroquine-resistant strain of Plasmodium falciparum and in 2 individuals before and during artificially induced fever. Plasma quinine levels were determined by both a benzene extraction method (QB), which measures principally unmetabolized quinine, and a metaphosphoric acid precipitation method (QMPA), which measures quinine and quinine metabolites. The ratio QB/QMPA in plasma was used to estimate the extent of metabolism of quinine. In all individuals plasma levels of quinien and QB/QMPA ratios were increased during malaria, suggesting impaired hepatic metabolism of quinine. The changes observed during malaria were not due to altered renal excretion of quinine. In 2 subjects in whom fever was artificially induced there were similar changes in quinine metabolism. These observations suggest that quinine dosage should be modified during the initial period of treatment, when symptoms and fever are greatest, in acute falciparum malaria.
Worden AN, Frape DL, Shephard NW. Consumption of quinine hydrochloride in tonic water. Lancet. 1987 Jan 31;1(8527):271-2. doi: 10.1016/s0140-6736(87)90087-0. PMID: 2880086.
REID AW, BECKER CH. The use of cocoa syrups for masking the taste of quinine hydrochloride. J Am Pharm Assoc Am Pharm Assoc. 1956 Mar;45(3):151-2. doi: 10.1002/jps.3030450307. PMID: 13319075.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (3)
Component type: Chemical Main substances: Last update: 2019-06-20 16:26:22 | Chemical Risk: |