Tiiips app ingredients
... live healthy!


Tiiips app ingredients
... live healthy!


| "Descrizione" by A_Partyns (12874 pt) | 2023-Dec-04 11:51 |
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
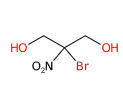
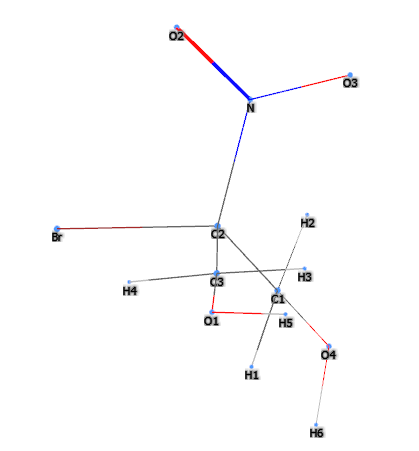
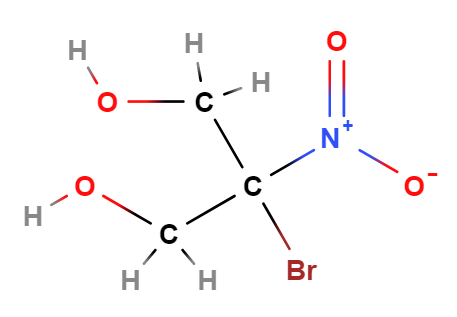
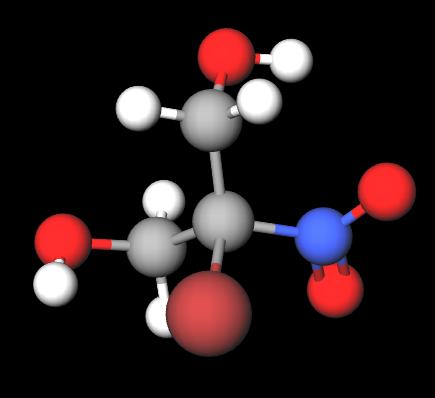
2-Bromo-2-Nitropropane-1,3-Diol (Bronopol) is a chemical compound, is a chemical compound mainly used as a preservative in various cosmetic products and for personal care. It consists of:

The name defines the structure of the molecule:
The synthesis process takes place in several stages:
It occurs as a fine white powder, easily soluble in water, alcohol, propylene glycol, ethyl acetate, slightly soluble in oil and insoluble in acetone and chloroform. Stable under acidic conditions. When the aqueous solution is alkaline, it decomposes slowly and cannot be used with certain metals such as aluminium. It releases formaldehyde. This study found that the decompodsition process producing formaldehyde mainly depends on temperature and pH. Notably, the pH of the Bronopol-diluted buffer solution was the factor most influencing the release of formaldehyde. The release was markedly higher in alkaline buffer compared with acidic buffer. (Kajimura, K., Tagami, T., Yamamoto, T., & Iwagami, S. (2008). The release of formaldehyde upon decomposition of 2-bromo-2-nitropropan-1, 3-diol (bronopol). Journal of health science, 54(4), 488-492.)

What it is used for and where
Cosmetics
It is a restricted ingredient V/21 as a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. This study warns of: "Actual formaldehyde levels from formaldehyde releasers were analysed using a modified HPLC method: Doi et al. detected > 30 mg/kg (> 30 ppm) in 83 of 89 cosmetic product samples containing different FRs, and > 250 ppm in 44/89 samples (3). As summarised in a review by de Groot et al. “… all releasers (with the exception of 2-bromo-2-nitropropane-1,3-diol, for which adequate data are lacking) can, in the right circumstances of concentration and product composition, release >200 ppm formaldehyde, which may result in allergic contact dermatitis.”(Bernauer, U., Bodin, L., Chaudhry, Q., Coenraads, P.J., Dusinska, M., Ezendam, J., Gaffet, E., Galli, C.L., Granum, B.B., Panteri, E. and Rogiers, V., 2021. SCCS SCIENTIFIC ADVICE ON on the threshold for the warning ‘contains formaldehyde’in Annex V, preamble point 2 for formaldehyde-releasing substances-SCCS/1632/21–Scientific advice.)
Preservative. Any product containing organic, inorganic compounds, water, needs to be preserved from microbial contamination. Preservatives act against the development of harmful microorganisms and against oxidation of the product.
First synthesised in 1897, since 1980 it has been used as an antibacterial and antifungal agent to prevent the growth and reproduction of bacteria in personal care products and as a broad-spectrum antibiofilm agent particularly in leave-on and rinse-off shampoos, creams, lotions, rinses and eye make-up. It is added in the formulation of cosmetics such as shampoos, conditioners and creams in a sterilising concentration of 0.01% - 0.02%.
Medical
Since 1960, it has been a broad-spectrum antibacterial and antibiofilm agent and a promising candidate for the treatment of chronic wounds (1).
Other Uses
Most significant studies
The aim of this study was to analyse the bacterial community in the production line of a calcium carbonate manufacturing company and to investigate possible causes of bacterial presence. A formulation containing 7.5-15% (v v -1 ) bronopol 1.0-2.5% (v v v -1 ) (chloroisothiazolinone (CIT) + methylisothiazolinone (MIT)) proved to be most effective. Of the possible causes of bacterial presence, sporogenesis and absorption of the biocide to the carbonate particles are the least likely compared to bacterial absorption of the particles and the acquisition of biocide resistance (2).
In terms of allergy, however, according to data from the 2015 - 2016 Pediatric Contact Dermatitis Registry, the top 10 allergens for children (0-5 years) include nickel (42%), peru balsam (19%) (15%), neomycin (17%), formaldehyde (15%), cocamidopropyl betaine (15%), cobalt dichloride (14%), MCI / MI (12%), propylene glycol (9%), bacitracin, bronopol and wool alcohol (8%) (3).
In recent years, Bronopol (2-bromo-2-nitropropane-1,3-diol), a broad-spectrum biocide, has seen use as an effective and economically acceptable alternative in the treatment and control of Saprolegnia sp. a fungus of the family Saprolegniaceae (4), however, concerns are growing regarding its use within industry due to its acute toxicity to several fish species and its potentially harmful effects on human health (5).
Typical commercial product characteristics Bronopol
| Appearance | White crystalline powder |
| Assay | 99.0% – 101.0% |
| Boiling Point | 358.0±42.0 °C at 760 mmHg |
| Melting Point | 130-133°C |
| Flash Point | 170.3±27.9°C |
| Density | 2.0±0.1 g/cm3 |
| pH | 5.0-7.0 |
| Impurity | <0.3% |
| Water | ≤0.5% |
| Sulphated ash | ≤0.1% |
| Water Solubility | 25 g/100 mL (22ºC) |
| PSA | 86.28000 |
| LogP | 1.72 |
| Refraction Index | 1.575 |
| Vapor Pressure | 0.0±1.8 mmHg at 25°C |
| Safety |  |
 |  |
 |  |
Synonyms:
References_________________________________________________________________
(1) Lee VE, O'Neill AJ. Potential for repurposing the personal care product preservatives bronopol and bronidox as broad-spectrum antibiofilm agents for topical application. J Antimicrob Chemother. 2019 Apr 1;74(4):907-911. doi: 10.1093/jac/dky520.
(2) Odić D, Prah J, Avguštin G. Identification of bacterial contaminants from calcium carbonate filler production lines and an evaluation of biocide based decontamination procedures. Biofouling. 2017 Apr;33(4):327-335. doi: 10.1080/08927014.2017.1310848.
(3) Goldenberg A, Mousdicas N, Silverberg N, Powell D, Pelletier JL, Silverberg JI, Zippin J, Fonacier L, Tosti A, Lawley L, Wu Chang M, Scheman A, Kleiner G, Williams J, Watsky K, Dunnick CA, Frederickson R, Matiz C, Chaney K, Estes TS, Botto N, Draper M, Kircik L, Lugo-Somolinos A, Machler B, Jacob SE. Pediatric Contact Dermatitis Registry Inaugural Case Data. Dermatitis. 2016 Sep-Oct;27(5):293-302. doi: 10.1097/DER.0000000000000214.
(4) Pottinger TG, Day JG. A Saprolegnia parasitica challenge system for rainbow trout: assessment of Pyceze as an anti-fungal agent for both fish and ova. Dis Aquat Organ. 1999 May 12;36(2):129-41. doi: 10.3354/dao036129.
(5) Piamsomboon P., Lukkana M., Wongtavatchai J. Safety and Toxicity Evaluation of Bronopol in Striped Catfish (Pangasianodon hypophthalmus) Thai. J. Vet. Med. 2013;43:477–481
Flores S, Montenegro I, Villena J, Cuellar M, Werner E, Godoy P, Madrid A. Synthesis and Evaluation of Novel Oxyalkylated Derivatives of 2',4'-Dihydroxychalcone as Anti-Oomycete Agents against Bronopol Resistant Strains of Saprolegnia sp. Int J Mol Sci. 2016 Aug 22;17(8):1366. doi: 10.3390/ijms17081366.
| Evaluate |