| "Descrizione" by Frank123 (12416 pt) | 2023-Apr-28 21:18 |
Review Consensus: 8 Rating: 8 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
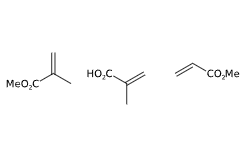
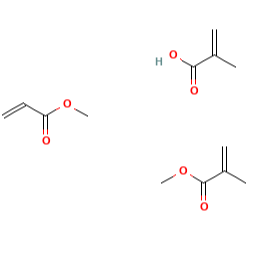
E1207 (Copolimero di metacrilato anionico) è un composto chimico prodotto in soluzione acquosa attraverso un processo di polimerizzazione con metacrilato di metile, acido metacrilico e acrilato di metile. Gli iniziatori radicali liberi sono polisorbato 80 e laurilsolfato di sodio. Quest'ultimo composto non risulta additivo alimentare autorizzato. Simethicone provvede a ridimensionare la schiuma.
Si presenta in forma di polvere bianca solubile in acqua.

A cosa serve e dove si usa
Alimentazione
Ingrediente inserito nella lista degli additivi alimentari europei come E1207 con funzione di agente di rivestimento negli integratori alimentari solidi ( capsule, pastiglie, compresse, pillole e altre forme simili come pellet e polveri).
Medicina
Copolimero di metacrilato anionico è utilizzato in microcapsule come rilascio di farmaco ad un tasso di ordine zero molto elevato
Sicurezza
Il Gruppo di esperti scientifici dell'EFSA sugli additivi alimentari e le fonti di nutrienti aggiunti agli alimenti ritiene che l'uso dell'AMC negli integratori alimentari solidi ai livelli d'uso e d'uso proposti non desti preoccupazioni per la sicurezza. Il gruppo di esperti scientifici non è stato in grado di valutare la sicurezza del copolimero di metacrilato anionico per l'uso in alimenti solidi a fini medici speciali (1).
 |  |
 |
- Formula molecolare C13H20O6
- Peso molecolare 272.29
- CAS 26936-24-3
- UNII 99Q3C7L77T
- EC Number
Bibliografia_____________________________________________________________________
(1) EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), 2010. Scientific Opinion on the safety of anionic methacrylate copolymer for the proposed uses as a food additive. EFSA Journal, 8(7), p.1656.
Abstract. The Panel on Food Additives and Nutrient Sources added to Food provides a scientific opinion on the use of anionicmethacrylate copolymer (AMC, a 30% dispersion of the dry copolymer in water) as a coating agent for solid foodsupplements and solid foods for special medical purposes (FSMPs). The dispersion contains 0.3% sodium laurylsulphate, which is not an authorised food additive. The opinion does not include a safety evaluation of thissubstance. From studies on toxicokinetics, acute and subchronic oral toxicity, genotoxicity, and developmentaltoxicity it is concluded that AMC is essentially not absorbed and that if any very low amounts of material wereabsorbed such material would not be retained in the tissues. No data on reproductive toxicity, chronic toxicity andcarcinogenicity are provided. In the absence of such data, chronic effects in the gastrointestinal tract following oraladministration cannot be excluded. Therefore, the Panel considers that an ADI should not be established, and that amargin of safety (MOS) approach is appropriate. Data from in vitro Ames and mammalian cell mutation assays andan in vivo micronucleus assay do not raise concern with respect to genotoxicity. A subchronic toxicity study and adevelopmental toxicity study in the rat provided NOAELs of respectively 1500 mg/kg bw/day (highest dose tested)and 1000 mg/kg bw/day (one dose tested). The anticipated exposure to AMC from both its use in food supplementsand in pharmaceuticals is 23.4 mg/kg bw/day for high consumer adults and 16 mg/kg bw/day for children. From the NOAELs, a coating level of 100 mg/tablet AMC and a combined exposure (food supplements and pharmaceuticals), MOS values between 43 to 64 for adults and 63 to 94 for children were calculated. The Panel considers these MOSsufficient given the lack of absorption and that the exposure estimates are based on worst case assumptions. The Panel concludes that the use of AMC in solid food supplements at the proposed use and use levels is not of safetyconcern. The Panel could not assess the safety of anionic methacrylate copolymer for uses in solid foods for specialmedical purposes.
| Evaluate |

