| "Descrizione" by Frank123 (12474 pt) | 2023-Apr-29 17:42 |
Review Consensus: 8 Rating: 8 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
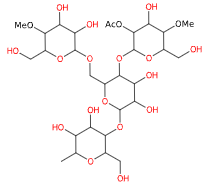
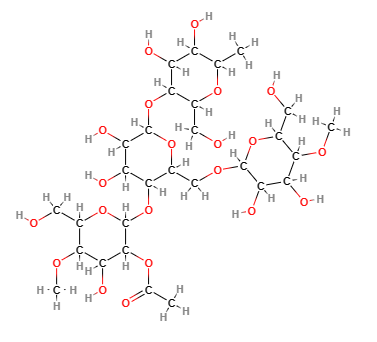
Acetylated starch is a modified starch produced by a process of cross-linked esterification of food starches (maize, rice) with vinyl acetate or acetic anhydride.
It appears as a white powder or white granules.

What it is used for and where
Food
Ingredient included in the list of European food additives as E1420 with the function of thickening and coating agent.
Medical
Acetylated starch has excellent encapsulation efficiency as a drug delivery agent (1).
Safety
The EFSA Panel on Food Additives and Nutrient Sources Added to Food considers that there is no safety concern for the use of modified starches as food additives at the uses and use levels declared for the general population and that a numerical ADI is not necessary (2).
 |  |
 |  |
- Molecular Formula C29H50O21
- Molecular Weight 734.7
- CAS 9045-28-7
- UNII
- EC Number 618-556-3
References_____________________________________________________________________
(1) Robert, P., García, P., Reyes, N., Chávez, J. and Santos, J., 2012. Acetylated starch and inulin as encapsulating agents of gallic acid and their release behaviour in a hydrophilic system. Food chemistry, 134(1), pp.1-8.
Abstract. Gallic acid (GA) was encapsulated with native starch (NS), native inulin (NIn), acetylated starch (AS) or acetylated inulin (AIn) (two substitution degrees each) by spray-drying, and a 22 statistical factorial design was used to evaluate each system. GA microparticles, produced under optimal conditions, were characterised by determining the GA encapsulation efficiency (ME) and their release profile in water. The inclusion of an acetyl group in the starch molecule improved the GA–ME. However, the opposite effect was observed for acetylated inulin. The release profile was fitted to First-order, Peppas and Higuchi models, and it was consistent with a non-Fickian diffusion (anomalous diffusion). No statistical differences were identified between the GA release rate constants for the GA-starch systems, whereas the acetylated inulin microparticles showed significantly lower GA release rate constants. The GA release pattern was fast for all systems studied (<9 h), suggesting that the microparticles could be used in the design of functional foods for dry mixes or instantaneous foods.
(2) EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), Mortensen, A., Aguilar, F., Crebelli, R., Di Domenico, A., Dusemund, B., Frutos, M.J., Galtier, P., Gott, D., Gundert‐Remy, U. and Lambré, C., 2017. Re‐evaluation of oxidised starch (E 1404), monostarch phosphate (E 1410), distarch phosphate (E 1412), phosphated distarch phosphate (E 1413), acetylated distarch phosphate (E 1414), acetylated starch (E 1420), acetylated distarch adipate (E 1422), hydroxypropyl starch (E 1440), hydroxypropyl distarch phosphate (E 1442), starch sodium octenyl succinate (E 1450), acetylated oxidised starch (E 1451) and starch aluminium octenyl succinate (E 1452) as food additives. EFSA Journal, 15(10), p.e04911.
Abstract. Following a request from the European Commission, the EFSA Panel on Food Additives and Nutrient sources added to Food (ANS) was asked to deliver a scientific opinion on the re-evaluation of 12 modified starches (E 1404, E 1410, E 1412, E 1413, E 1414, E 1420, E 1422, E 1440, E 1442, E 1450, E 1451 and E 1452) authorised as food additives in the EU in accordance with Regulation (EC) No 1333/2008 and previously evaluated by JECFA and the SCF. Both committees allocated an acceptable daily intake (ADI) ‘not specified’. In humans, modified starches are not absorbed intact but significantly hydrolysed by intestinal enzymes and then fermented by the intestinal microbiota. Using the read-across approach, the Panel considered that adequate data on short- and long-term toxicity and carcinogenicity, and reproductive toxicity are available. Based on in silico analyses, modified starches are considered not to be of genotoxic concern. No treatment-related effects relevant for human risk assessment were observed in rats fed very high levels of modified starches (up to 31,000 mg/kg body weight (bw) per day). Modified starches (e.g. E 1450) were well tolerated in humans up to a single dose of 25,000 mg/person. Following the conceptual framework for the risk assessment of certain food additives, the Panel concluded that there is no safety concern for the use of modified starches as food additives at the reported uses and use levels for the general population and that there is no need for a numerical ADI. The combined exposure to E 1404–E 1451 at the 95th percentile of the refined (brand-loyal) exposure assessment scenario for the general population was up to 3,053 mg/kg bw per day. Exposure to E 1452 for food supplement consumers only at the 95th percentile was up to 22.1 mg/kg bw per day.
| Evaluate |
