| "Descrizione" by Frank123 (12474 pt) | 2024-Sep-22 18:46 |
Review Consensus: 10 Rating: 10 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Sodium alginate is a chemical compound, a linear unbranched amorphous copolymer, the sodium salt of alginic acid, a natural polysaccharide usually extracted from brown algae. It is biodegradable and biocompatible.
The name defines the structure of the molecule:
- Sodium is a chemical element with the symbol Na (from the Latin "Natrium") and the atomic number 11. It is a soft, silvery-white, highly reactive metal and is an alkaline metal.
- Alginate refers to a type of polysaccharide derived from algae. Alginates are known for their ability to form gels and are often used as thickeners, stabilizers or emulsifiers.
The synthesis process takes place in several stages:
- Collection. Brown algae are harvested from the ocean and the species of algae used may vary, but often include types such as Laminaria, Ascophyllum and Macrocystis.
- Washing and drying. Seaweed is thoroughly washed to remove salt, sand and other marine debris and dried to reduce water content.
- Alkaline treatment. Dried seaweed is treated with an alkali, usually sodium carbonate. This breaks the cell walls of algae and releases alginate.
- Extraction. The algae are then heated in an alkali solution. This causes the alginate to dissolve in the solution, forming a dense, viscous liquid.
- Precipitation. The alginate solution is precipitated using an acid, usually hydrochloric acid or sulfuric acid. This causes the alginate to solidify and separate from the solution.
- Conversion. The precipitated alginate is converted into its sodium salt form by treating the alginate with sodium carbonate.
- Purification. Sodium alginate is purified to remove any remaining impurities. This usually involves dissolving the alginate in water, filtering the solution and subsequent precipitation of the alginate.
- Drying. In the last step, the purified sodium alginate is dried and ground into a fine powder suitable for use.
It appears as a white powder.

What it is used for and where
Food
Ingredient on the European food additives list as E401 with various functions: thickener, gelling agent, emulsifier.
In the food industry, it is also used as a preservative to reduce microbial spoilage in certain foods (1).
Safety
EFSA: Following a request from the European Commission, the Panel on Dietetic Products, Nutrition and Allergies provided a scientific opinion on 'sodium alginate and Ascophyllum nodosum' in relation to the reduction of post-prandial glycaemic responses. The Panel considers that sodium alginate with an M/G ratio of 1.50 is sufficiently characterised in relation to the effects attributed. The question is: can "alginate reduce the activity of digestive enzymes and reduce glucose absorption"? The panel of experts believes that the reduction in post-prandial glycaemic responses (provided post-prandial insulinemic responses are not disproportionately increased) may be a beneficial physiological effect (2).
Medical
In the pharmaceutical industry, it's used to help form gels in the production of drugs and in wound dressings.
Sodium alginate is used in the treatment of heartburn, peptic ulcer and to counteract gastro-oesophageal reflux. It is also used to prevent crystallisation of pills or tablets by acting as a plasticising agent. (3).
Cosmetics
Sodium alginate is used not in its pure form, but as ALGIN, SODIUM ALGINATE SULFATE, SODIUM ALGIN SULFATE, SODIUM/TEA-UNDECYLENOYL ALGINATE and others. Functions vary, but are generally humectant.
Other uses
- Polyelectrolyte used as an aqueous binder in sodium ion batteries.
- In textile printing, it's used as a thickener for the paste containing the dye.
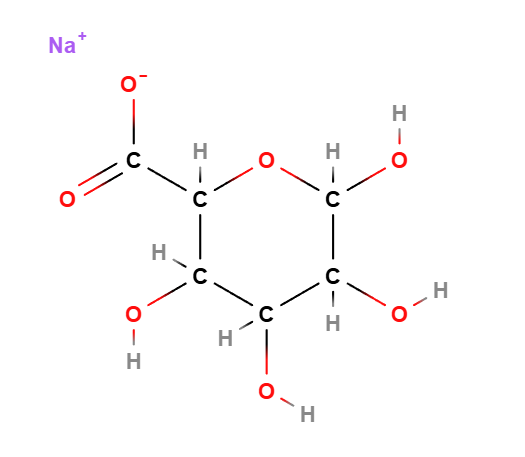 |  |
Formula molecolare C6H9NaO7
Peso molecolare 216.12
CAS 9005-38-3
UNII C269C4G2ZQ
EC Number 618-415-6
IUPAC sodium;(2S,3R,4S,5R)-3,4,5,6-tetrahydroxyoxane-2-carboxylate
InChl=1S/C6H10O7.Na/c7-1-2(8)4(5(10)11)13-6(12)3(1)9;/h1-4,6-9,12H,(H,10,11);/q;+1/p-1/t1-,2+,3+,4-,6?;/m0./s1
InChl Key MSXHSNHNTORCAW-KSSASCOMSA-M
SMILES C1(C(C(OC(C1O)O)C(=O)[O-])O)O.[Na+]
MDL number MFCD00081310
FEMA 2015
NACRES NA.21
RTECS AZ5820000
NCI C84148
RXCUI 56446
Nikkaji J2.064.428A.
References_____________________________________________________________________
(1) Heydari R, Bavandi S, Javadian SR Effect of sodium alginate coating enriched with horsemint (Mentha longifolia) essential oil on the quality of bighead carp fillets during storage at 4°C.. Food Sci Nutr. 2015 May
Abstract. Effect of sodium alginate coating enriched with horsemint essential oil (HEO) on the quality of bighead carp (Aristichthys nobilis) fillets at refrigeration temperature (4 ± 1°C) was studied. Bighead carp fillets were coated with neat sodium alginate (SA) and sodium alginate containing 0.5 and 1% v/v of HEO and their quality changes in terms of total volatile basic nitrogen (TVB-N), peroxide value (PV), thiobarbituric acid (TBA), and microbial counts were investigated. SA coating enriched with the essential oil could reduce the spoilage of the fillets and extend their shelf-life. Samples treated with SA-containing HEO showed significantly (P < 0.05) lower TVB-N content and lipid oxidation, as reflected by lower PV, FFA and TBA values during the storage period compared with the SA and control. The treatment also reduced the degree of microbial deterioration of the fillets (about 1.5 log10 CFU/g) more efficiently than the SA.
(2) Scientific Opinion on the substantiation of health claims related to sodium alginate and reduction of post prandial glycaemic responses (ID 1868, 1881) pursuant to Article 13(1) of Regulation (EC) No 1924/2006 - EFSA Journal 2011;9(6):2261 [15 pp.].
(3) Borba PA, Pinotti M, de Campos CE, Pezzini BR, Stulzer HK. Sodium alginate as a potential carrier in solid dispersion formulations to enhance dissolution rate and apparent water solubility of BCS II drugs. Carbohydr Polym. 2016 Feb
Qiang, T., Wang, J., Jiang, L., & Xiong, K. (2022). Modulation of hyperglycemia by sodium alginate is associated with changes of serum metabolite and gut microbiota in mice. Carbohydrate Polymers, 291, 119359
Long, R., Yu, Z., Shan, M., Feng, X., Zhu, X., Li, X. and Wang, P., 2022. The easy-recoverable 3D Ni/Fe-LDH-SA gel ball encapsulated by sodium alginate is used to remove Ni2+ and Cu2+ in water samples. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 634, p.127942.
Xu, H., Jiang, K., Zhang, X., Zhang, X., Guo, S. and Zhou, H., 2019. Sodium alginate enabled advanced layered manganese-based cathode for sodium-ion batteries. ACS applied materials & interfaces, 11(30), pp.26817-26823.
Abstract. Sodium-ion batteries (SIBs) are promising candidates applied to large-scale energy storage systems owing to abundant sodium resources and high economic efficiency. Layered manganese-based oxides as a prevailing cathode for sodium-ion batteries have been extensively studied, where doping or coating has been demonstrated to improve the electrochemical performance. However, the binder that tends to be the popular poly(vinylidene difluoride), is revealed to generate swellability upon cycling, leading to electrode material cracks and disconnection with current collectors. For the above issues, in this work, environmentally friendly sodium alginate is utilized as the aqueous binder in a conventional layered transition-metal oxide cathode P2-Na2/3MnO2 for SIBs. Through credible comparative experiments, sodium alginate is testified to play an essential role in suppressing cracks on the surface of materials, preventing surge in charge-transfer resistance and restraining detachment between electrode and current collector. Therefore, sodium alginate is proved to be an ideal binder to match with P2-Na2/3MnO2, where some issues existed before, as a promising cathode material with excellent performance and low cost. This study displays that improving battery performance by exploring suitable binder systems can equal or even exceed the performance improvement through modification of the material itself, and this perspective of enhancement should not be ignored.
| Evaluate |
