![]() Panax japonicus
Panax japonicus
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
0 pts from admin
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about Panax japonicus by admin (19534 pt) | 2025-Dec-06 19:01 |
| Read the full Tiiip | (Send your comment) |
Panax japonicus
Description
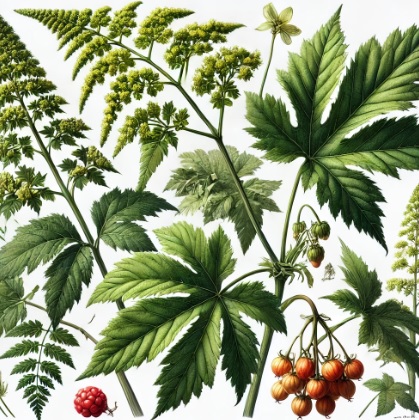
Panax japonicus is a perennial herbaceous species belonging to the botanical family Araliaceae, characteristic of montane forest understoreys in East Asia. It develops a fleshy, branched rhizome, which represents the most important part from an herbal and pharmacognostic point of view, and an upright aerial stem usually bearing a single whorl of leaves.
The leaves are palmately compound, composed of 3–7 ovate–lanceolate leaflets with serrated margins and a rather leathery texture; their colour ranges from medium to deep green, often darker in shade-adapted populations. The plant forms a terminal umbel with numerous small whitish to greenish flowers. The fruit is a bright red drupe, similar to that of Panax ginseng but generally smaller. Growth is slow, and the species depends on shaded conditions, humus-rich soils and constant, moderate moisture.
 |  |
Botanical classification (APG IV system)
| Category | Data |
|---|---|
| Common name | Japanese ginseng |
| Botanical name | Panax japonicus C.A.Mey. |
| Kingdom | Plantae |
| Clade | Angiosperms → Eudicots → Asterids |
| Order | Apiales |
| Family | Araliaceae |
| Genus | Panax |
| Species | Panax japonicus C.A.Mey. |
Indicative nutritional values per 100 g (dried root of Panax japonicus)
Values may vary depending on age of the root, cultivation area, and drying process.
| Component | Approximate value per 100 g |
|---|---|
| Energy | ~ 290–320 kcal |
| Total carbohydrates | ~ 60–65 g |
| — of which sugars | ~ 5–8 g |
| Dietary fiber | ~ 18–22 g |
| Proteins | ~ 6–8 g |
| Total lipids | ~ 2–3 g |
| — of which saturated fatty acids (SFA) | ~ 0.3–0.5 g |
| — monounsaturated fatty acids (MUFA) | ~ 0.2 g |
| — polyunsaturated fatty acids (PUFA) | ~ 0.5–0.7 g |
| Sodium | very low |
| Main minerals | potassium, calcium, magnesium (moderate amounts) |
| Vitamins | trace levels (mainly water-soluble) |
Lipid profile note
The dried root contains a low lipid fraction. Saturated fatty acids (SFA, saturated fatty acids), monounsaturated fatty acids (MUFA, mono-unsaturated fatty acids), and polyunsaturated fatty acids (PUFA, poly-unsaturated fatty acids, including both n-6 and n-3) are all present only in very small quantities and have negligible dietary impact.
Distribution, Ecology And Cultivation Aspects
Panax japonicus is native to Japan, eastern China, Korea and Taiwan, where it colonises mountain forest understoreys typically between mid to high elevations. It prefers:
Cool, moist forests with a well-developed canopy;
Deep, well-drained soils enriched with organic matter;
Shaded or semi-shaded exposures, being sensitive to direct, intense sunlight;
Moderate temperatures, with good tolerance to cold but sensitivity to persistent heat or drought.
Cultivation is demanding because the species requires stable microclimatic conditions and protected, shaded sites. Propagation can be achieved by division of the rhizome or by seed, the latter often showing pronounced dormancy and slow germination. To obtain high-quality rhizomes, multi-year growth (typically 4–7 years or more) is required, with careful management of soil moisture and protection against fungal diseases and root rot.
Chemical Composition And Rhizome Characteristics
The rhizome of Panax japonicus contains a complex mixture of triterpenoid saponins, commonly referred to as chikusetsusaponins, structurally related to ginsenosides of Asian ginseng but with distinct profiles. These saponins are considered the main bioactive constituents of the species.
In addition, the rhizome also contains:
Polysaccharides with relatively high molecular weight;
Polyacetylenes and phytosterols;
Amino acids and trace elements.
The qualitative and quantitative profile of saponins and other constituents is influenced by plant age, ecological conditions, and post-harvest processing (washing, slicing, drying parameters).
Traditional Uses And Modern Applications
In East Asian traditional medicine, Panax japonicus has been used for centuries as a tonic and “qi-regulating” herb, often compared to Panax ginseng, although its ethnopharmacological identity and phytochemistry are distinct. It has been traditionally employed to:
Support general vitality and resistance to fatigue;
Help maintain physiological resilience to stress;
Contribute to circulatory and hemostatic balance;
Promote overall wellbeing and functional balance of the organism.
Dried rhizomes are used in decoctions, herbal teas, powders and extracts, alone or in combination with other medicinal plants. Recent experimental research has explored potential antioxidant, anti-inflammatory, metabolic and vascular effects of chikusetsusaponins and related compounds, although clinical evidence remains limited and further studies are needed to clarify efficacy and safety in humans.
Rhizome Quality And Commercial Requirements
Key criteria for assessing the quality of Panax japonicus rhizomes include:
External appearance: uniform colour, absence of mould, cracks, insect damage or pronounced defects;
Size and weight of rhizomes, which reflect plant age and proper development;
Total saponin content and specific marker compounds (selected chikusetsusaponins), crucial for standardisation;
Residual moisture maintained within appropriate limits to ensure stability and shelf-life;
Botanical purity, excluding contamination with other Panax species or unrelated roots;
Microbiological quality in line with standards for herbal raw materials and preparations.
Accurate control of harvesting time (often in late growing season), drying conditions (temperature, ventilation, duration) and storage (light, humidity, packaging) is essential to preserve the chemical integrity and functional value of the rhizome.
Production process
Plant material and harvesting
Cultivation in shaded, well-drained mountain or hilly areas, typically in East Asia (China, Japan).
Plants are usually grown for 3–5 years before harvest to allow sufficient accumulation of triterpenoid saponins in rhizomes and roots.
Harvesting of rhizomes and roots in the appropriate season (often autumn), when the saponin content is at or near its maximum.
Primary processing of raw material
Careful washing to remove soil and foreign matter.
Optional cutting of rhizomes/roots into slices or segments.
Drying in controlled conditions (air-drying or low-temperature dryers) to reach a stable moisture level suitable for storage and extraction.
Selection, removal of damaged material, and packaging of the dried root/rhizome.
Extraction process (for herbal and cosmetic use)
Milling or coarse grinding of dried plant material.
Solvent extraction using water, ethanol, or hydroalcoholic mixtures under controlled time, temperature, and drug-to-solvent ratio.
Filtration of the extract and vacuum concentration to obtain a dense extract.
Drying (e.g., spray-drying or vacuum drying) to yield a dry extract standardized in saponins.
Possible standardization by blending with inert carriers to achieve a constant content of total saponins or specific markers (e.g., chikusetsusaponin IVa, ginsenoside Ro).
Quality control includes chromatographic profiling (HPLC/UPLC), assay of total saponins, microbiological limits, heavy metals, pesticide residues, and residual solvents where applicable.
Applications
Herbal and dietary supplement field
Used in traditional East Asian medicine (Chinese, Japanese Kampo) primarily as:
A general tonic and support in fatigue and reduced vitality.
A component in formulations with immunomodulatory and anti-inflammatory intent.
Modern formulations typically include:
Dry standardized extracts in capsules or tablets.
Liquid extracts and tinctures.
Functional products where botanicals are permitted (shots, tonics, herbal blends).
Food context
Panax japonicus is not a staple food and is mainly present as a herbal ingredient.
It can appear in:
Herbal teas and decoctions.
Tonifying beverages or nutraceutical-type products, where allowed by local regulations.
Cosmetic field
Extracts (commonly Panax Japonicus Root Extract) are incorporated into:
Facial care products with claims of tonifying, protective, or anti-age support.
Products aimed at stress-exposed skin and supportive antioxidant protection.
Scalp and hair formulations (lotions, shampoos) designed to support scalp condition and hair vitality, usually in synergy with other active ingredients.
All uses and associated claims must comply with national legislation on botanicals, health claims, and cosmetics.
Nutrition & health
At typical supplement doses, the energy contribution is negligible; interest focuses on bioactive components.
Experimental and preclinical studies on P. japonicus total saponins and chikusetsusaponins report:
Antioxidant and anti-inflammatory activities.
Immunomodulatory and organ-protective effects, including liver- and kidney-protective actions in animal models.
Potential roles in metabolic modulation (lipids, glucose) and anti-obesity effects through inhibition of fat absorption and pancreatic lipase in animal models.
Possible neuroprotective actions via modulation of oxidative stress and mitochondrial pathways (preclinical data).
Clinical evidence in humans remains limited and does not justify specific therapeutic claims; P. japonicus should be considered a supportive botanical within a healthy lifestyle, not a replacement for medical treatment.
In patients with chronic diseases or under medication (e.g., anticoagulants, antidiabetics, antihypertensives), medical advice is recommended before use.
Portion note
There is no standard food portion for Panax japonicus.
In commercial products, typical daily intakes are:
A few grams of dried root in herbal teas or decoctions (often in multi-herb blends).
Hundreds of milligrams per day of standardized dry extract in food supplements, according to manufacturer specifications.
Dosages must always follow label instructions and, where relevant, professional recommendations.
Allergens and intolerances
Panax japonicus is not classified as a major allergen in standard food legislation.
Possible reactions:
Individual hypersensitivity with gastrointestinal discomfort, headache, agitation, or skin reactions in predisposed subjects (by analogy with other Panax species).
Potential interactions (based largely on data from Panax spp. in general):
With anticoagulant/antiplatelet medications (theoretical influence on hemostasis).
With antidiabetic drugs (possible modulation of glycemia).
With antihypertensive treatments (possible influence on blood pressure and heart rate).
In multi-ingredient supplements, allergen risk typically derives from other ingredients or excipients (soy, milk, gluten, etc.) and must be verified on the label.
Storage and shelf-life
Dried root/rhizome
Store in a cool, dry place, protected from direct light and heat sources.
Use closed containers (multi-layer bags, lined cartons, rigid containers) to minimize moisture uptake and contamination.
Typical shelf-life: 2–3 years under appropriate conditions and with compliant microbiological quality.
Dry extracts
Store in hermetic, preferably opaque containers under dry conditions; avoid strong temperature fluctuations.
Limit exposure to humidity and oxygen, which may promote degradation of saponins and oxidation of sensitive constituents.
Typical shelf-life: 24–36 months, depending on purity, excipients, packaging, and storage conditions.
Safety and regulatory aspects
Use of Panax japonicus in food supplements is subject to national rules on permitted botanicals, maximum levels if any, and safety requirements.
In some jurisdictions it is primarily recognized as a traditional medicinal plant, which may affect how it can be marketed.
Production must follow GMP (Good Manufacturing Practices) and HACCP (Hazard Analysis and Critical Control Points), with controls on:
Botanical identity (correct species, absence of adulteration/substitution).
Purity (absence of foreign matter and contaminant plants).
Contaminants: heavy metals, pesticides, mycotoxins within legal limits.
Microbiological quality appropriate for herbal raw materials and extracts.
Residual solvents in extracts compliant with pharmacopeial or food-supplement regulations.
Any nutrition and health claims must comply with applicable regulations (e.g., EU Regulation (EC) No 1924/2006 and national guidance on botanicals and claims).
Labeling
For herbal and supplement products, labels may include:
Name of the herbal material:
“Panax japonicus root” or “Panax japonicus rhizome”.
“Panax japonicus extract” for extracts.
For standardized extracts:
Drug-to-extract ratio (e.g., 10:1).
Stated content of total saponins or specific markers (e.g., chikusetsusaponin IVa, ginsenoside Ro).
Extraction solvent (e.g., ethanol/water).
For cosmetic products:
Typical INCI: Panax Japonicus Root Extract.
Additional required information:
Batch/lot number, best-before or expiry date.
Storage conditions.
Directions for use and warnings (e.g., not recommended in pregnancy or lactation without medical advice; do not exceed the recommended daily dose; keep out of reach of children), according to national rules.
INCI functions (cosmetics)
Typical cosmetic designation: Panax Japonicus Root Extract
Main functions:
Skin conditioning: Supports maintenance of skin in good general condition.
Tonifying/protective role: Used in products for tired or stressed skin, often in synergy with other antioxidant plant extracts.
Antioxidant/anti-age co-adjuvant: Contributes to strategies aimed at mitigating oxidative-stress-related damage.
Scalp care: Included in lotions and shampoos intended to support scalp condition and hair vitality, usually in combination with other actives.
Formulators must consider pH range, solvent system, compatibility, and stability of the extract within the cosmetic base.
Conclusion
Panax japonicus is an Araliaceae species of significant interest in herbal medicine, dietary supplements, and certain cosmetic applications.
Its rhizomes and roots are a rich source of triterpenoid saponins, including chikusetsusaponins and ginsenosides, which have been associated in preclinical studies with antioxidant, anti-inflammatory, metabolic, and organ-protective activities.
When identity control, extraction procedures, analytical standardization, and storage conditions are properly managed under GMP/HACCP, Panax japonicus can provide standardized and safe raw materials for use in regulated food supplements and cosmetic formulations, always within the limits of current evidence and legal frameworks.
Mini-glossary
Triterpenoid saponins: Glycosides of triterpenoid aglycones; in Panax japonicus they are major bioactive constituents with diverse pharmacological activities demonstrated mainly in preclinical models
Chikusetsusaponins: A group of dammarane-type triterpenoid saponins characteristic of P. japonicus, used as chemical markers in quality control and associated with several biological effects in experimental studies.
Standardized dry extract: A dry preparation obtained after extraction and drying, adjusted to contain a defined amount of one or more marker constituents (e.g., total saponins).
GMP – Good Manufacturing Practices: A set of practices ensuring consistent, controlled production and quality of products, from raw material handling to finished goods.
HACCP – Hazard Analysis and Critical Control Points: A systematic approach to identifying, evaluating, and controlling hazards that are significant for food and supplement safety.
References__________________________________________________________________________
Wang T, Huang Y, Hu M, Huang X, Yu D, Jia L, Zhi W, Mu Y, Zhou Z, Wang J. Saponins from Panax japonicus Enhance Lipolysis via Acting on FGF21-β-Klotho/FGFR1 in Obese Mice. J Agric Food Chem. 2025 Jun 25;73(25):15624-15636. doi: 10.1021/acs.jafc.5c01012.
Abstract. Saponins from Panax japonicus (SPJ) had a significant antiobesity action, and fibroblast growth factor 21 (FGF21) plays a central role in energy, lipid, and glucose homeostasis. We explored whether FGF21 is the target site of SPJ in lipolysis to improve lipid metabolism. We established an obese mouse model that was fed with a high-fat diet (HFD) for 16 weeks, and these obese mice were medicated with low-dose SPJ (15 mg/kg) or high-dose SPJ (45 mg/kg). The effects of SPJ on lipid metabolism, particularly on sympathetic activation and subsequent lipolysis in white adipose tissue (WAT), were evaluated. Furthermore, the impacts of SPJ on adipose FGF21 and its receptors β-Klotho (KLB) and FGFR1 within the central nervous system (CNS) were examined. Then, we observed the actions of FGF21 on WAT lipolysis as well as on KLB and FGFR1 within the CNS. Our results showed that SPJ treatment ameliorated lipid metabolism and protected against chronic HFD-induced obesity in a dose-independent manner. In WATs of HFD-induced obese mice, SPJ stimulated sympathetic innervation and enhanced lipolysis by increasing the expressions of FGF21 and its receptors (KLB and FGFR1). Moreover, SPJ had the capability to activate KLB- and FGFR1-expressing neurons in the paraventricular nucleus (PVN) of the hypothalamus and area postrema (AP)/nucleus tractus solitarius (NTS) of the HFD-induced obese mice. Interestingly, FGF21 analogue treatment partly recapitulated the action of SPJ on lipid metabolism by enhancing sympathetic activation and lipolysis in WATs of the HFD-induced obese mice. Like SPJ, FGF21 analogue treatment also activated KLB- and FGFR1-expressing neurons in PVN and AP/NTS. This study demonstrated that SPJ acted on adipose FGF21 and brain KLB/FGFR1 in obese mice to enhance sympathetic innervation and lipolysis, contributing to improved lipid metabolism.
Huang Y, Ning WY, Wang CE, Lin PC, Wang M, Meng XH. Research progress on chemical composition, pharmacological activity and clinical application of Panax japonicus. Nat Prod Res. 2025 Dec 1:1-13. doi: 10.1080/14786419.2025.2595534.
Abstract. Panax japonicus (T.Nees) C. A. Mey var. major (Burkill) C. Y. Wu & K. M. Feng is a plant of the Panax L. genus in the Araliaceae family. The medicinal part is the rhizome of P. japonicus, which is a traditional medicine used by ethnic minorities such as the Naxi, Yi, Tibetan etc. It has the effects of tonifying the lungs, nourishing yin, stopping bleeding, and activating collaterals, and is widely used in medicine, pharmaceuticals, and health products. The main chemical components of P. japonicus are triterpenoid saponins, as well as phenolic acids, polysaccharides, etc. It has various pharmacological activities such as anti-tumor, cardiovascular and cerebrovascular protection, antioxidant, and liver protection. To better develop and utilise the plant resources of P. japonicus and clarify its pharmacological substance basis, this article reviews the chemical composition, pharmacological activity, clinical application, and quality control of P. japonicus, in order to provide theoretical basis for in-depth research of P. japonicus.
Matsuda H, Samukawa K, Fukuda S, Shiomoto H, Tung CN, Kubo M. Studies of Panax japonicus fibrinolysis. Planta Med. 1989 Feb;55(1):18-21. doi: 10.1055/s-2006-961767.
Abstract. The effects of a 70% methanol extract (PMe) obtained from the rhizomes of Panax japonicus C. A. Meyer on experimental thrombosis and fibrinolysis were investigated in vivo and in vitro. PMe showed a promotive effect on the activation of the fibrinolytic system as determined by the euglobulin lysis time (ELT) assay but was inactive to the inhibitory effect against endotoxin-induced disseminated intravascular coagulation (DIC) in rats. PMe and its major components, chikusetsusaponin III, IV, and V, strongly promoted the action of urokinase in fibrin plate. These results suggested that PMe promotes the fibrinolysis and its effective components are chikusetsusaponin III, IV, and V.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Hardiness: Hardiness: Natural fertilizer: Hazards/diseases: Last update: 2025-01-17 15:46:18 | Sun exposure: Family: Commercial fertilizer: Main substances contained: |